A kind of preparation method of 1-n-ethyl gentamicin c1a
A technology of ethyl gentamycin and ethyl gentamycin, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of low yield, difficult separation and purification, cumbersome process, etc. , to achieve the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
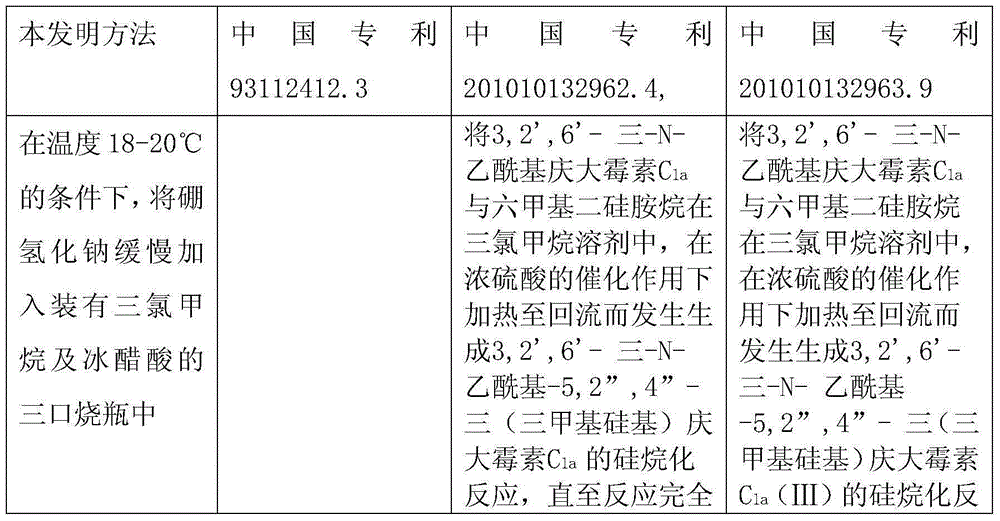
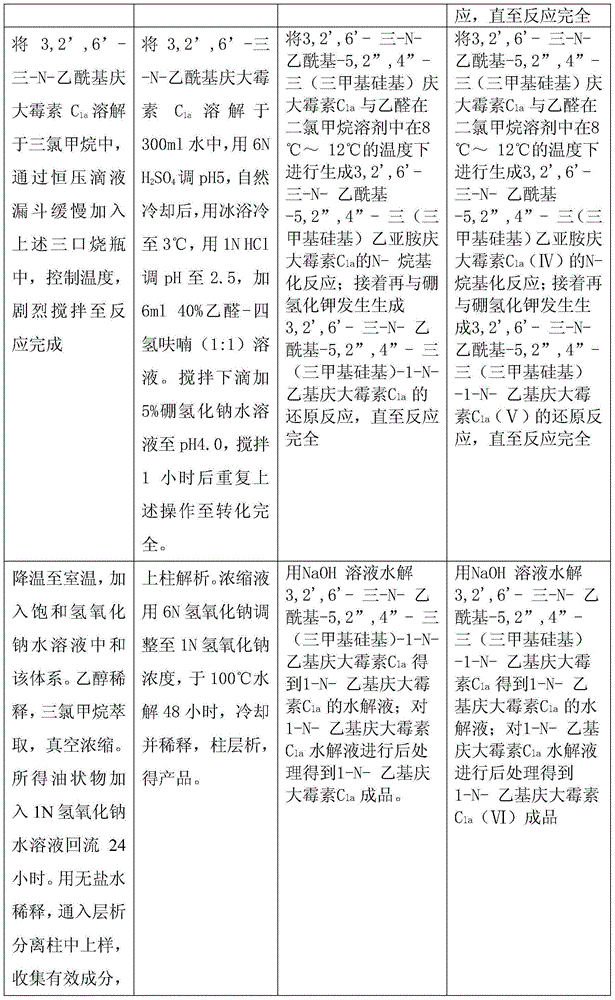
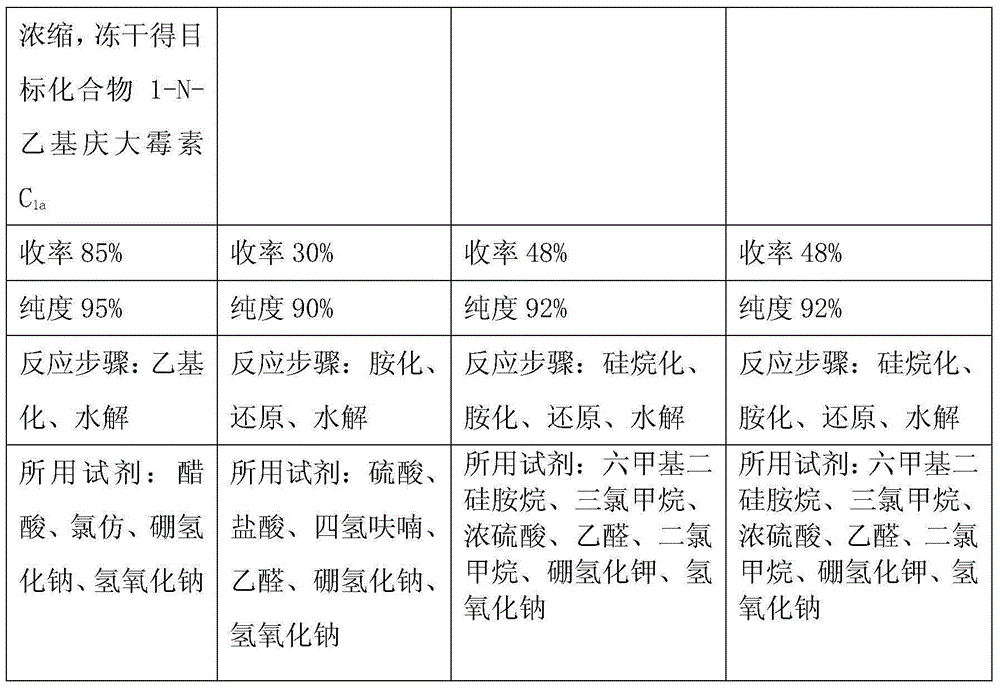
[0020] At a temperature of 18-20°C, slowly add 288g of sodium borohydride into a three-necked flask filled with 4L of chloroform and 2L of glacial acetic acid. 575g of 3,2',6'-tri-N-acetyl gentamicin C 1a Dissolve it in 6L of chloroform, slowly add it into the above-mentioned three-necked flask through a constant pressure dropping funnel, keep the temperature at 40°C, and vigorously stir for 20 hours. The temperature was lowered to 25°C, and saturated aqueous sodium hydroxide solution was added to neutralize the system. Add 5L of ethanol to dilute, extract three times with 3L of chloroform, and concentrate in vacuo. The obtained oil was added to 4 L of 1N aqueous sodium hydroxide solution and refluxed for 24 hours. Dilute it with 6L of non-salt water, pass it into a chromatographic separation column to load the sample, wash with non-salt water, analyze with ethanol aqueous solution, and collect the effective components. Concentrate and freeze-dry to obtain 396g of the produ...
Embodiment 2
[0022] At a temperature of 18-20°C, slowly add 330 g of sodium borohydride into a three-necked flask filled with 4 L of chloroform and 2 L of glacial acetic acid. 575g of 3,2',6'-tri-N-acetyl gentamicin C 1a Dissolve it in 6L of chloroform, slowly add it into the above-mentioned three-necked flask through a constant pressure dropping funnel, keep the temperature at 50°C, and vigorously stir for 20 hours. The temperature was lowered to 25°C, and saturated aqueous sodium hydroxide solution was added to neutralize the system. Add 5L of ethanol to dilute, extract three times with 3L of chloroform, and concentrate in vacuo. The obtained oil was added to 4 L of 1N aqueous sodium hydroxide solution and refluxed for 24 hours. Dilute it with 6L of non-salt water, pass it into a chromatographic separation column to load the sample, wash with non-salt water, analyze with ethanol aqueous solution, and collect the effective components. Concentrate and freeze-dry to obtain 405g of the pr...
Embodiment 3
[0024] At a temperature of 18-20°C, slowly add 300 g of sodium borohydride into a three-necked flask filled with 4 L of chloroform and 2 L of glacial acetic acid. 575g of 3,2',6'-tri-N-acetyl gentamicin C 1a Dissolve it in 6L of chloroform, slowly add it into the above-mentioned three-neck flask through a constant pressure dropping funnel, keep the temperature at 45°C, and vigorously stir for 20 hours. The temperature was lowered to 25°C, and saturated aqueous sodium hydroxide solution was added to neutralize the system. Add 5L of ethanol to dilute, extract three times with 3L of chloroform, and concentrate in vacuo. The obtained oil was added to 4 L of 1N aqueous sodium hydroxide solution and refluxed for 24 hours. Dilute it with 6L of non-salt water, pass it into a chromatographic separation column to load the sample, wash with non-salt water, analyze with ethanol aqueous solution, and collect the effective components. Concentrate and freeze-dry to obtain 419g of the prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com