Continuous chromatographic separation technology of gentamycin C1a
A continuous chromatography and gentamicin technology, which is applied in the field of separation and purification of high-purity gentamicin C1a recovered liquid, can solve problems such as difficult removal, achieve high resin utilization, compact system, and reduce labor load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
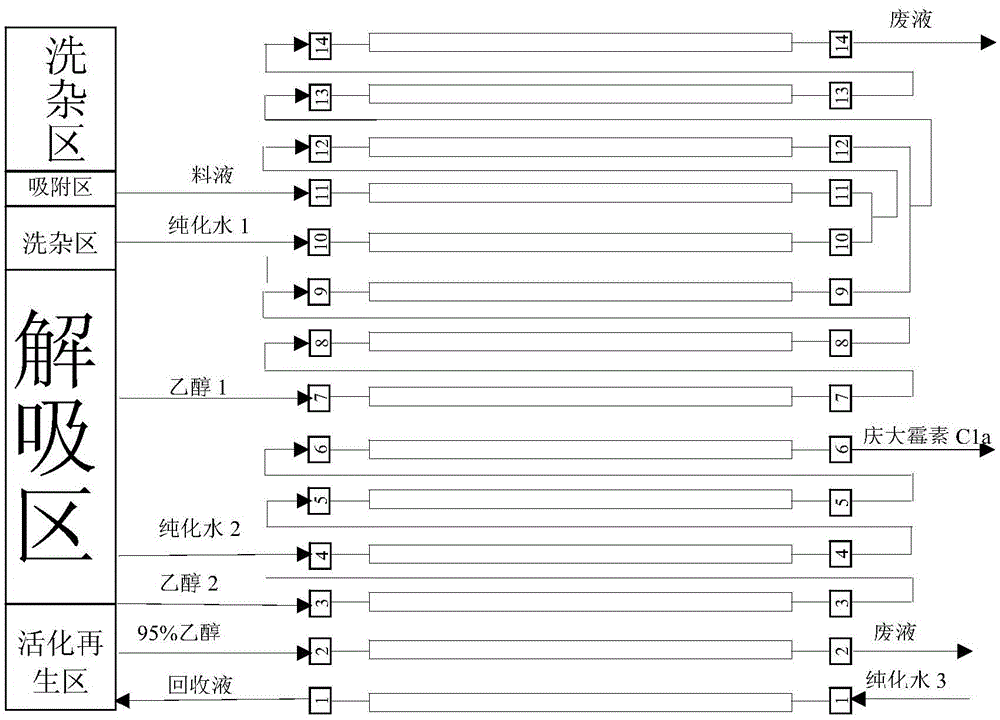
[0045] Combine below figure 1 And the embodiment is described in detail:
[0046] The selected resin of the present invention is No. 1 resin of Huazhen chromatography, and the resin particle diameter is more than 95% of 100 orders, and the filling capacity of each resin column is 0.08m 3 , the capacity of the resin column is 0.1m 3 , the actual filling ratio is 80%. The total size of the system is 3m×3m×5m (length×width×height).
[0047] The disc transfer type continuous chromatographic separation system separates gentamicin C1a into the following areas:
[0048] 1) Adsorption area: (Unit 11)
[0049] There is 1 unit (unit 11) in this area. Through flow rate control, the raw material first enters unit 11, and then enters unit 12 through series connection for cleaning.
[0050] 2) Laundry area: (Units 10, 12-14)
[0051]After adsorption, each resin needs to be washed with water, located before and after the adsorption zone. After the resin column rotates to the adsorptio...
Embodiment 2
[0066] Combine below figure 1 And the embodiment is described in detail:
[0067] The selected resin of the present invention is YPR-II resin, and the resin is 80 mesh, and the filling capacity of each resin column is 0.08m 3 , the capacity of the resin column is 0.1m 3 , the actual filling ratio is 80%. The total size of the system is about 3m×3m×5m (length×width×height).
[0068] The simulated moving bed continuous chromatographic separation system separates gentamicin C1a into the following areas:
[0069] 1) Adsorption area: (Unit 11)
[0070] There is 1 unit (unit 11) in this area. Through flow rate control, the raw material first enters unit 11, and then enters unit 12 through series connection for cleaning.
[0071] 2) Laundry area: (Units 10, 12-14)
[0072] After adsorption, each resin needs to be washed with water, located before and after the adsorption zone. After the resin column rotates to the adsorption water washing area, the material liquid (mainly clar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com