Method for preparing Etimicin sulfate
A technology of etimicin sulfate and dilute sulfuric acid, applied in the field of medicine, can solve the problems of low total yield, low reaction selectivity, complicated separation procedures, etc., and achieves optimization of reaction conditions and separation conditions, shortening of synthesis process, synthesis of The effect of process shortening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, preparation method of the present invention
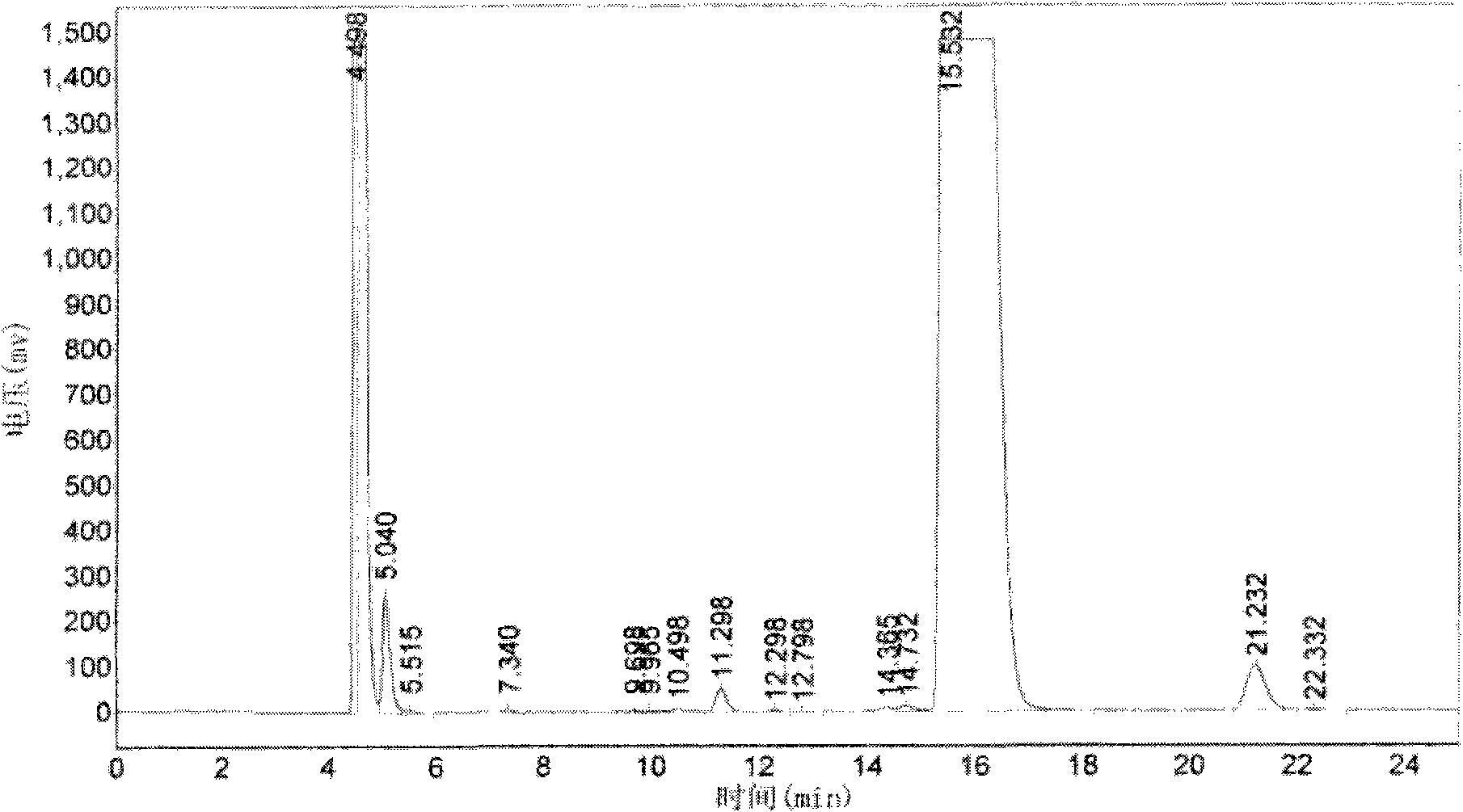
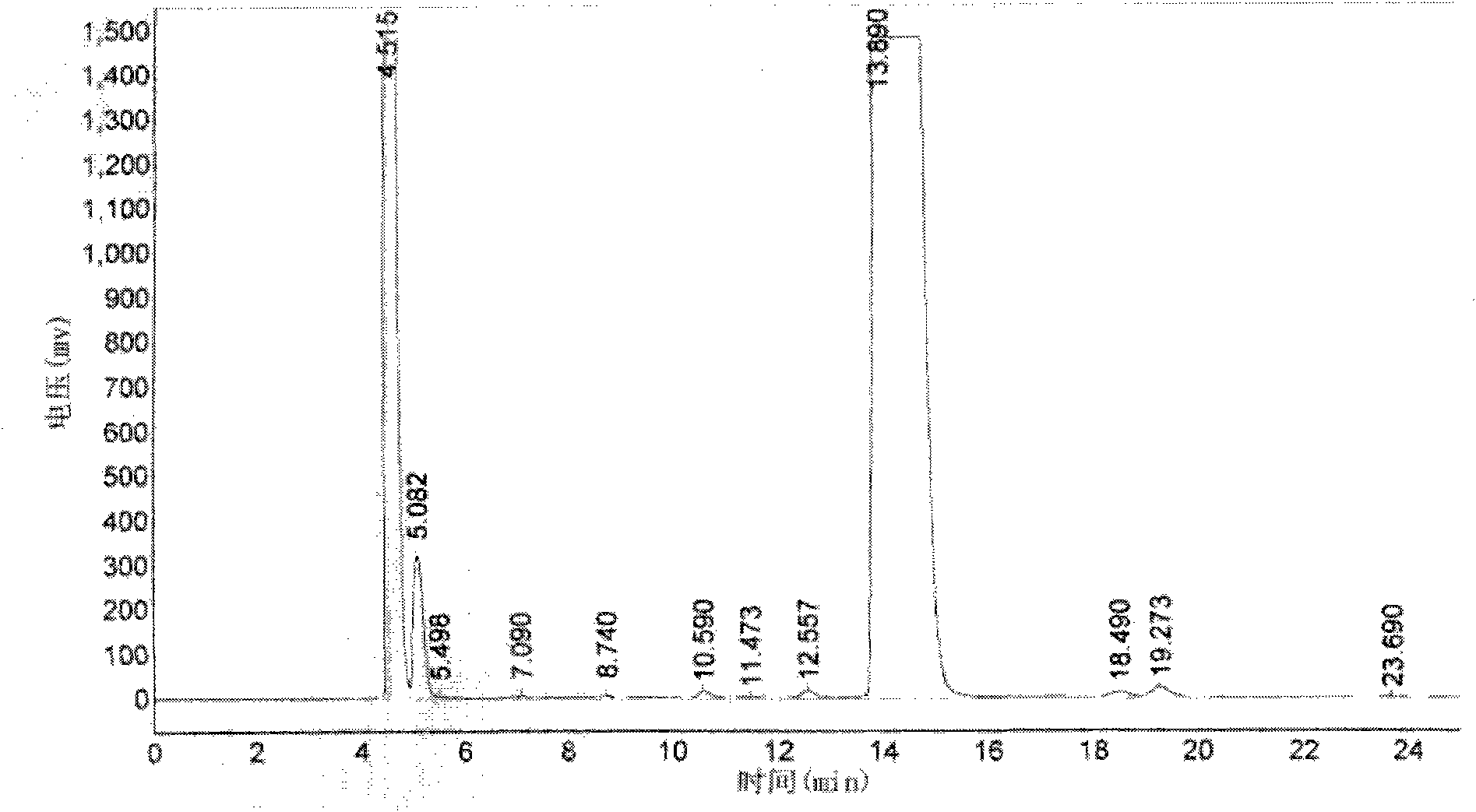
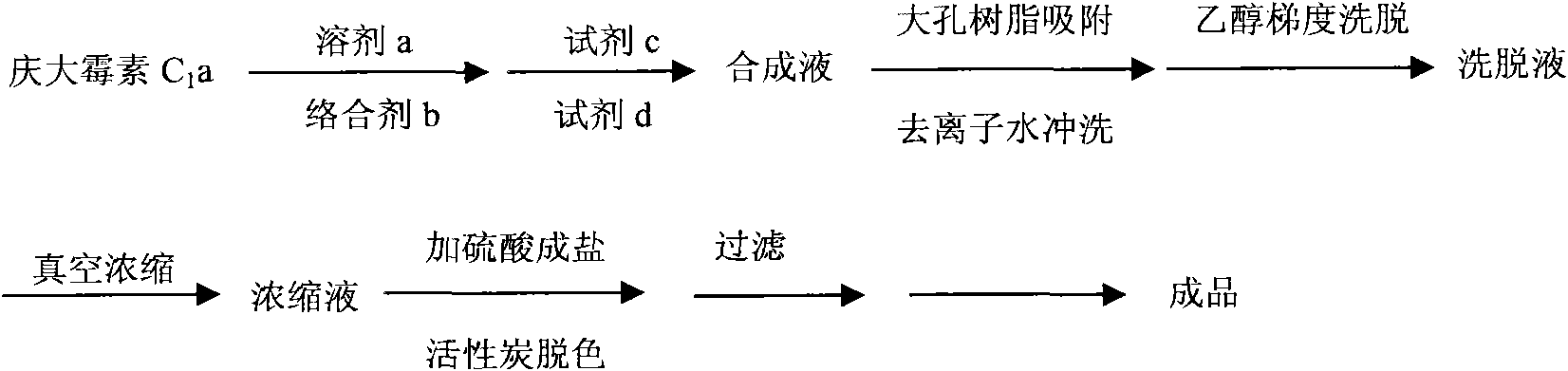
[0046] Take 10kg of gentamicin C1a base, add 30kg of solvent DMSO, stir and dissolve, add cobalt sulfate to the solution, stir and cool down to 14°C at the same time. Ethyl p-toluenesulfonate was added to the obtained mixture, stirred and reacted at 12°C for 2.5 hours, the reaction solution was added with reagent sodium borohydride, reacted for 1.5 hours, and then the pH was adjusted to 12.0 with 3 mol / L ammonia water. The obtained reaction solution was adsorbed with macroporous resin, and washed with deionized water until there was no solvent smell. The washed macroporous resin was eluted with 20% ethanol, and the purity of etimicin in the eluate was detected by HPLC, and the eluate with a purity greater than 90% was collected. The obtained eluate was concentrated in vacuo to recover ethanol, and the recovered ethanol was adjusted to the previous step after adjusting its concentration. Add 15% activated carb...
Embodiment 2
[0047] Embodiment 2, preparation method of the present invention
[0048]Take 15kg of gentamicin C1a base, add 40kg of solvent methanol, stir and dissolve, add cobalt acetate to the solution, stir and cool down to 13°C at the same time. Diethyl carbonate was added to the obtained mixture, stirred and reacted at 13° C. for 2.8 hours, sodium borohydride was added to the reaction solution, reacted for 2 hours, and then the pH was adjusted to 12.5 with 4 mol / L ammonia water. The obtained reaction solution was adsorbed with macroporous resin, and washed with deionized water until there was no solvent smell. The washed macroporous resin was eluted with 25% ethanol, and the purity of etimicin in the eluate was detected by HPLC, and the eluate with a purity greater than 90% was collected. The obtained eluate was concentrated in vacuo to recover ethanol, and the recovered ethanol was adjusted to the previous step after adjusting its concentration. Add 18% activated carbon to the obta...
Embodiment 3
[0049] Embodiment 3, preparation method of the present invention
[0050] Take 12kg of gentamicin C1a base, add 50kg of solvent acetonitrile, stir and dissolve, add N-Boc to the solution, stir and cool down to 10°C at the same time. Add acetaldehyde to the obtained mixture, stir and react at 13.5° C. for 3 hours, add potassium borohydride to the reaction solution, react for 2.5 hours, and then adjust the pH to 13.5 with 4.5 mol / L ammonia water. The obtained reaction solution was adsorbed with macroporous resin, and washed with deionized water until there was no solvent smell. The washed macroporous resin was eluted with 18% ethanol, and the purity of etimicin in the eluate was detected by HPLC, and the eluate with a purity greater than 90% was collected. The obtained eluate was concentrated in vacuo to recover ethanol, and the recovered ethanol was adjusted to the previous step after adjusting its concentration. Add 10% activated carbon to the obtained concentrated solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com