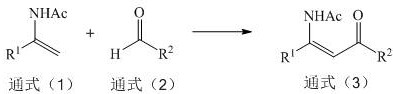
Electrochemical synthesis method of beta-acetamido carbonyl compound
A technology of carbonyl compounds and acetamido, which is applied in the field of electrochemical synthesis of β-acetamidocarbonyl compounds, can solve the problems of expensive catalyst preparation, complex reactions, low yield, and few types of substrates, so as to speed up electron transfer Effects of speed, high yield, and improved utilization of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] In a 25.0mL three-necked reaction flask, add 246.95mg tetrabutylammonium tetrafluoroborate as electrolyte, 5.0mL acetonitrile as reaction solvent, stir and dissolve, add 48.3mg 1-(acetylamino)-1-styrene and 54.045mg formic acid Methyl ester compound, use platinum sheet (15mm×15mm×0.3mm) as the anode-platinum sheet (15mm×15mm×0.3mm) as the cathode, insert it into the reaction bottle, open the reaction kettle, connect the circuit, turn on the power supply, and adjust the current to 10mA, keep the current constant, and react at 50°C for 6 hours. After the reaction is completed, the solvent is spin-dried and separated and purified by column chromatography to obtain the obtained product. The distance between the anode and the cathode is 3 cm.
[0021]Structural confirmation data: Yield 87%. was obtained as white solid . 1 H NMR (400 MHz, CDCl 3 ) δ 10.61 (s, 1H), 7.40 – 7.35 (m, 5H), 5.29 (s, 1H), 3.77 (s, 3H), 2.17(s, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ = 169...
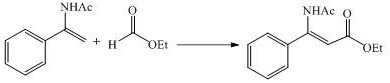
Embodiment 2
[0024]
[0025] In a 25.0mL three-necked reaction flask, add 263.41mg tetrabutylammonium tetrafluoroborate as electrolyte, 5.0mL acetonitrile as reaction solvent, stir and dissolve, add 48.3mg 1-(acetylamino)-1-styrene and 66.67mg formic acid Ethyl ester compound, use platinum sheet (15mm×15mm×0.3mm) as the anode-platinum sheet ((15mm×15mm×0.3mm) as the cathode, insert into the reactor, open the reactor, connect the circuit, turn on the power, and turn on the current Adjust it to 10mA, keep the current constant, and react at 50°C for 6 hours. After the reaction, spin the solvent to dry, and separate and purify it by column chromatography. The distance between the anode and the cathode is 3cm.
[0026] Structure confirmation data: Yield85%.was obtained as colorless oil. 1 H NMR (400 MHz, Chloroform- d ) δ 10.64 (s, 1H), 7.49 – 7.29 (m, 5H), 5.28 (s, 1H), 4.22 (q, J = 7.1 Hz, 2H), 2.16 (s, 3H), 1.32 (t, J = 7.2 Hz, 3H). 13 C NMR (100 MHz, CDCl 3 )δ = 168.70, 168.52, 154...
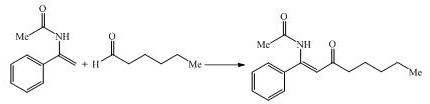
Embodiment 3
[0029]
[0030] In a 25.0mL three-necked reaction flask, add 117.49mg Ammonium perchlorate was used as the electrolyte, 5.0 mL of acetonitrile / water (V=4:1) was used as the reaction solvent, after stirring and dissolving, 48.3 mg of 1-(acetylamino)-1-styrene and 127.44 mg of benzaldehyde were added, and the graphite rod ((ϕ6mm) is used as the anode, platinum sheet ((15mm×15mm×0.3mm) is inserted into the reaction bottle as the cathode, open the reaction kettle, connect the circuit, turn on the power, adjust the current to 15mA, keep the current constant, and react at room temperature 5 hours, after the reaction was completed, the reaction was quenched with water, the reaction solution was extracted twice with ethyl acetate, the organic phases were combined and washed with saturated brine, finally dried with anhydrous magnesium sulfate, filtered, spin-dried, and separated by column chromatography Purified, that is, the distance between the anode and the cathode is 3cm.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com