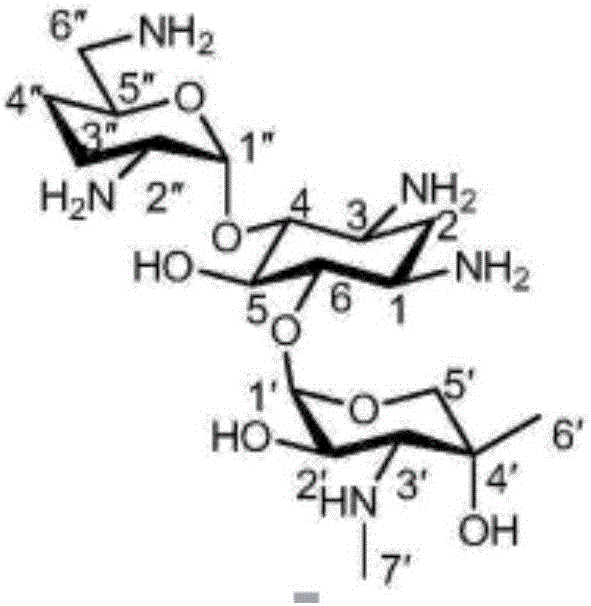
Preparation method for gentamicin C1a
A technology of gentamicin and seeds, which is applied in the field of preparation of gentamicin C1a, can solve the problems of cumbersome operation and high production cost, and achieve the effect of stable output and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of gentamicin C1a comprises the following steps.
[0030] Step (1), carry out microwave mutagenesis with gentamicin B (the mutant of Micromonospora aculeatus) producing bacterium, draw 2mL spore liquid and be suspended in EP tube, EP tube is placed in ice-bath beaker, in Treat in a microwave oven at 700W, 2450MHz for 10s; then use a chemical mutagen for chemical mutagenesis, the chemical mutagen is nitrosoguanidine, and the concentration of the mutagen is 0.4mg / mL, at 20°C, in a constant temperature incubator Constant temperature cultivation was carried out for 6 h. Gentamicin C1a-producing strains were screened.
[0031] Step (2), selecting stable and relatively high-yielding gentamicin C1a-producing bacteria, and performing slant culture in slant medium. The components in the slant culture medium are (unit: g / L): cornstarch 1.8, KNO 3 0.05, NaCl0.04, MgSO 4 0.03,K 2 HPO 4 0.02, wheat flour 1.8, calcium carbonate 0.09, agar 1.7, and the bal...
Embodiment 2
[0040] The preparation method of gentamicin C1a comprises the following steps.
[0041] Step (1), carry out microwave mutagenesis to gentamicin B producing bacteria, draw 2mL spore liquid and suspend in EP tube, put EP tube in ice bath beaker, process 30s with 700W, 2450MHz in microwave oven; Then use A chemical mutagen is used for chemical mutagenesis, the chemical mutagen is nitrosoguanidine, the mutagen is used at a concentration of 0.7 mg / mL, and a constant temperature culture is carried out in a constant temperature incubator at 30° C. for 7 hours. Gentamicin C1a-producing strains were screened.
[0042] Step (2), selecting stable and relatively high-yielding gentamicin C1a producing bacteria, and carrying out slant culture. The components in the slant medium are (unit: g / L): corn starch 2.0, KNO 3 0.1, NaCl0.05, MgSO 4 0.05,K 2 HPO 4 0.03, wheat flour 2.0, calcium carbonate 0.10, agar 2.0, and the balance is water. The pH of the slant medium was natural (pH7±0.2). ...
Embodiment 3
[0051] The preparation method of gentamicin C1a comprises the following steps.
[0052] Step (1), carry out microwave mutagenesis to gentamicin B producing bacteria, draw 2mL spore liquid and suspend in EP tube, put EP tube in ice-bath beaker, process 60s with 700W, 2450MHz in microwave oven; Then use A chemical mutagen was used for chemical mutagenesis, the chemical mutagen was nitrosoguanidine, and the concentration of the mutagen was 1.0 mg / mL, and the culture was carried out in a constant temperature incubator at 38° C. for 8 hours. Gentamicin C1a-producing strains were screened.
[0053] Step (2), select stable and relatively high-yielding gentamicin C1a producing bacteria, carry out slant culture, the components in the slant medium are (unit: g / L): cornstarch 2.2, KNO 3 0.15, NaCl0.06, MgSO 4 0.07,K 2 HPO 4 0.04, wheat flour 2.2, calcium carbonate 0.11, agar 2.3, and the balance is water. The pH of the slant medium was natural (pH7±0.2). Cultured at 40°C for 15 day...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com