Preparation method of 1-N-ethyl gentamicin Cla
A technology of ethyl gentamicin and gentamicin is applied in the field of preparation of medicinal raw materials, can solve the problem of low yield and the like, and achieves the advantages of avoiding alkylation side reactions, enhancing polarization and avoiding hydrolysis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
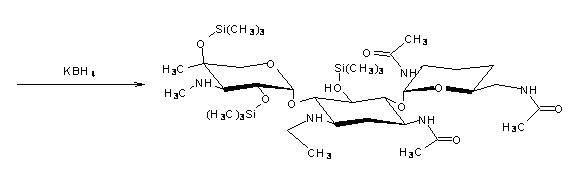
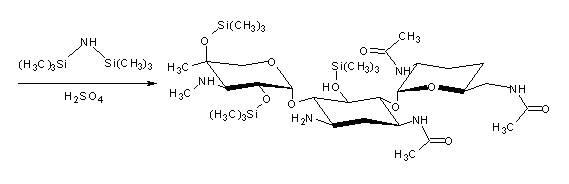
[0029] ①Add 100mL of chloroform solvent, 20mL of 0.104mol hexamethyldisilazane and 0.05mL of catalytic concentrated sulfuric acid (98wt%) into the dried three-neck flask with reflux device, and then add 15g 0.026mol of 3,2',6'-tris-N-acetylgentamycin C 1a (I), heated to reflux to generate 3,2',6'-tri-N-acetyl-5,2",4"-tris(trimethylsilyl)gentamycin C 1a (Ⅱ) The silanization reaction, until the reaction is complete, the time of heating and reflux is 5h. Then, the chloroform solvent was evaporated to dryness to obtain 3,2',6'-tri-N-acetyl-5,2",4"-tris(trimethylsilyl)gentamycin C 1a (Ⅱ).
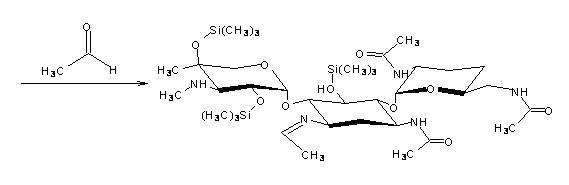
[0030] ②Reduce the temperature of the system in step ① to 20°C~25°C, add 100mL of dichloromethane, continue to cool down to 10°C, add 1.5mL of acetaldehyde, and generate 3,2',6'-tri-N- Acetyl-5,2",4"-tris(trimethylsilyl)ethylimine Gentamicin C 1a (Ⅲ) N-alkylation reaction for 1h. Then add 20mL of boric acid buffer solution with a pH of 9 to 10 (the boric acid buffer solution is composed of ...
Embodiment 2~ Embodiment 8)
[0035] The preparation method of each embodiment is basically the same as that of Example 1, except that the hexamethyldisilazane used in step ① and 3,2',6'-tri-N-acetylgentamycin C 1a The mol ratio, the mol ratio of each embodiment and the impact on reaction are shown in Table 1.
[0036] Table 1
[0037]
Hexamethyldisilazane and 3,2',6'-tri-N-acetylgentamycin C 1a molar ratio
3,2',6'-tri-N-acetylgentamycin C 1a conversion rate of
Example 1
4.0∶1
70.2%
Example 2
1.5∶1
43.8%
Example 3
2∶1
48.5%
Example 4
2.5∶1
53.2%
Example 5
3∶1
60.1%
Example 6
3.5∶1
69.7%
Example 7
4.5∶1
69.9%
Example 8
5∶1
68.4%
[0038] Theoretically, 1 mol of hexamethyldisilazane can silylate 2 mol of alcoholic hydroxyl groups, and every 1 molecule of 3,2',6'-tri-N-acetylgentamycin C 1a There are 3 alcoholic hydroxyl groups, therefore, hexamethyldisilazane and 3,2',6'-tri-N-...
Embodiment 9~ Embodiment 12)
[0041] The preparation method of each embodiment is basically the same as that of Example 1, except that the acetaldehyde used in step 2 and the 3,2',6'-tri-N-acetylgentamycin C used in step 1 1a The mol ratio, the mol ratio of each embodiment and the impact on reaction are shown in Table 2.
[0042] Table 2
[0043]
Acetaldehyde and 3,2',6'-tris-N-acetylgentamycin C 1a molar ratio
3,2',6'-tri-N-acetylgentamycin C 1a conversion rate of
ETM-1
ETM-2
Example 1
1∶1
70.2%
0.7%
1.5%
Example 9
0.8∶1
65.4%
0.4%
1.0%
Example 10
1.2∶1
70.3%
3.1%
8.2%
Example 11
1.5∶1
68.5%
4.2%
10.1%
Example 12
2∶1
69.5%
4.3%
13.4%
[0044] As can be seen from Table 2, increase the consumption of acetaldehyde, although the transformation rate improves slightly, two by-reaction products ETM-1 (3 "-N-ethylgentamycin C 1a ) and ETM-2 (1,3”-di-N-ethylgentamycin C 1a ) is also ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com