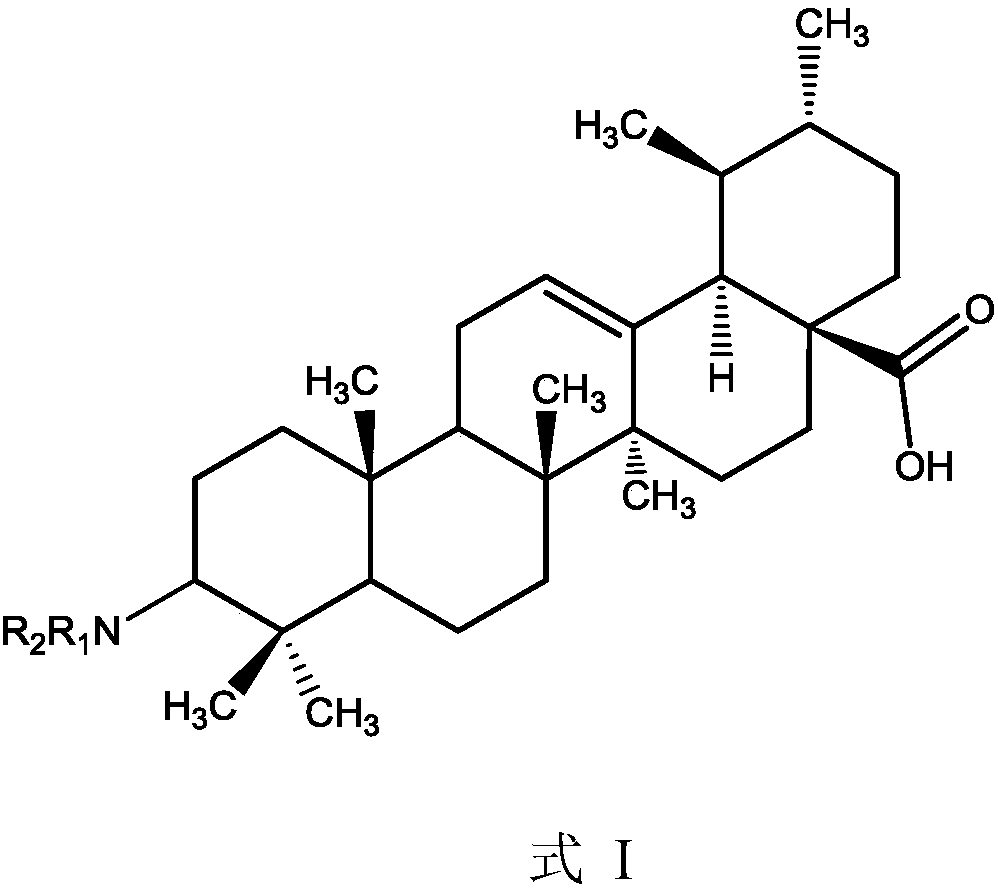
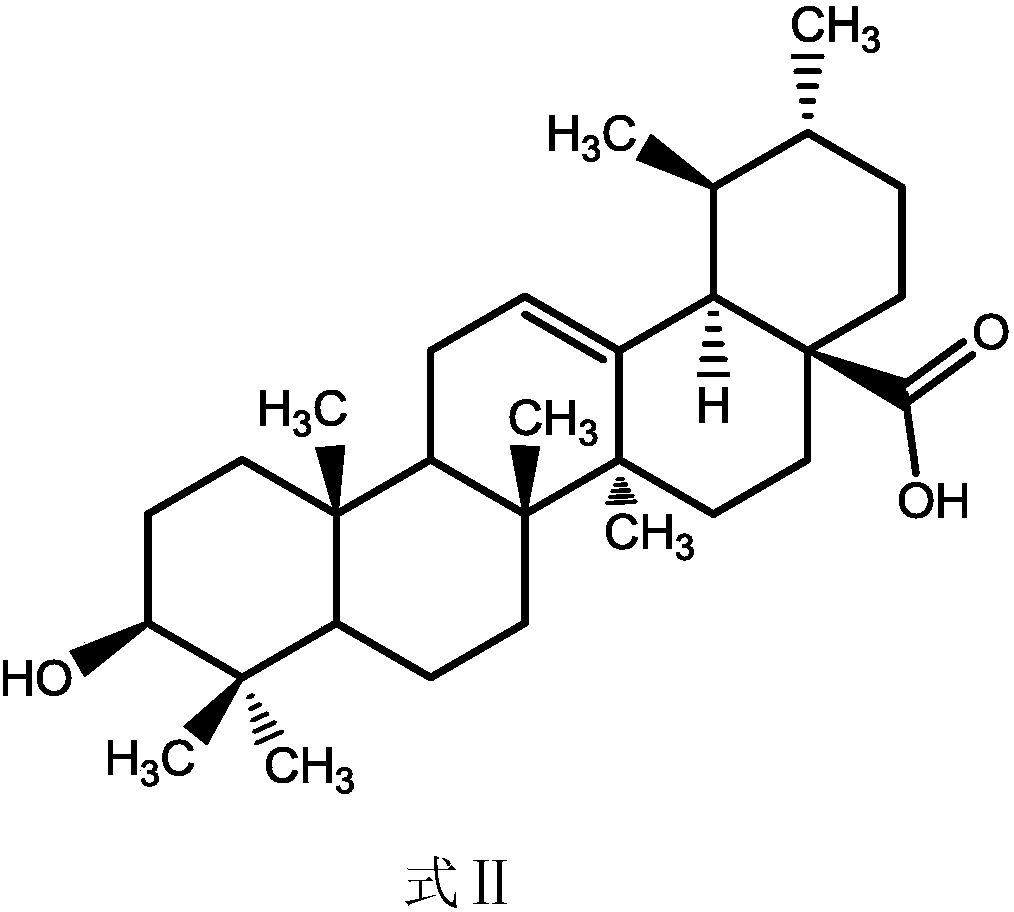
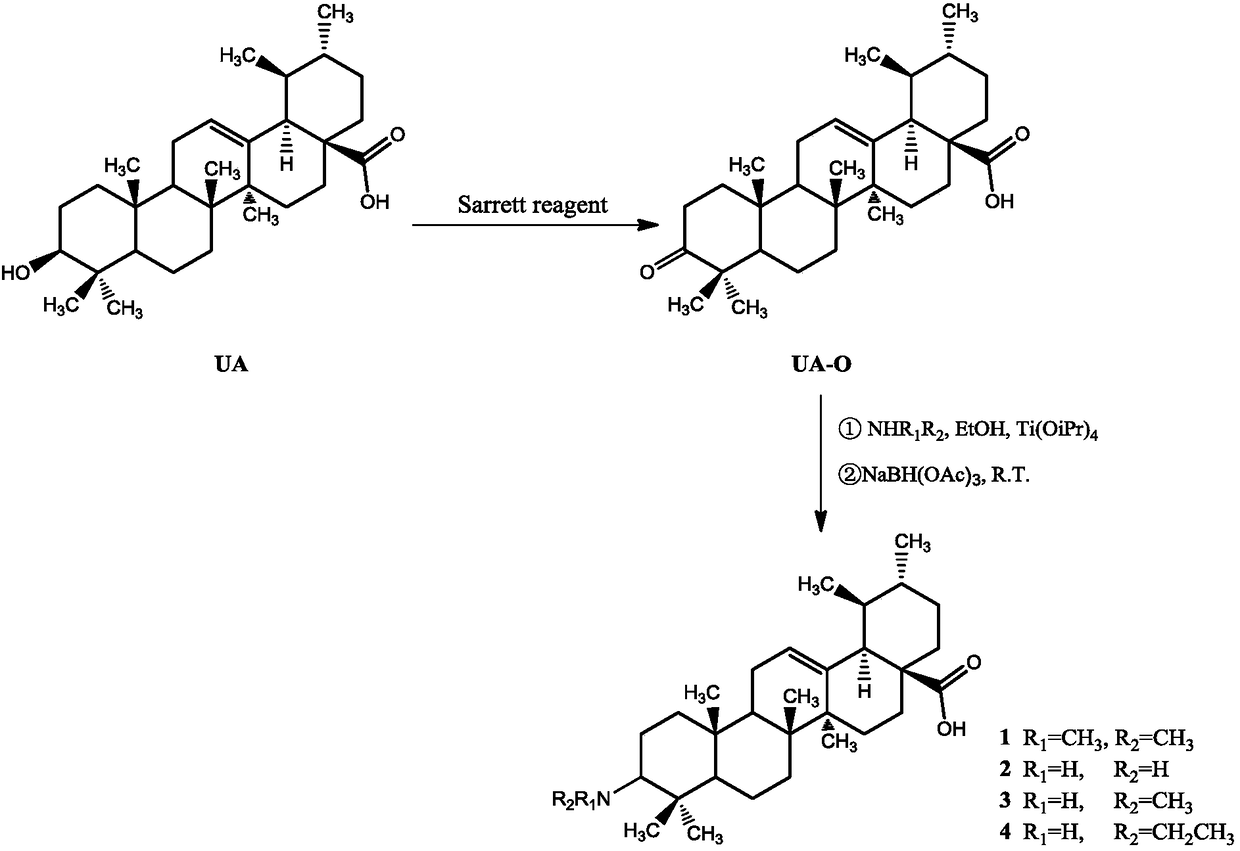
Ursolic acid derivative, preparation method thereof, and application thereof in preparation of drug for treating MRSA infection
A technology of ursolic acid and derivatives, applied in antibacterial drugs, steroids, organic chemistry, etc., can solve the problems of enhanced druggability, insoluble in water, and the activity needs to be further improved, and achieves rapid reaction and selectivity. Strong, water-soluble improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 0.91g (2.0mmol) of ursolic acid (98.6%) and add it to a 100mL round bottom flask, add 50mL of dichloromethane, stir well until it is completely dissolved, add 0.11g (5.0mmol) of Sarrite reagent, and stir at 10°C for reaction 8h, add pure water to quench the reaction, separate the liquid in a separatory funnel, extract the aqueous layer once more with dichloromethane, combine the dichloromethane extracts, and pass through a silica gel chromatography column (dichloromethane:ethyl acetate, 5:1) Separation and purification to obtain ursolic acid C 3 Oxidation product of hydroxyl group (UA-O) 0.74g, purity 98.2%.
[0017] Take 0.14g UA-O (0.30mmol) into a 50mL three-necked flask, add ammonia-saturated absolute ethanol solution to fully dissolve, add 0.50mL isopropyl titanate solution under the protection of argon, and stir at 25°C for 6 hours. Then add 0.063g (0.3mmol) sodium triacetoxyborohydride and react for 2h, then quench the reaction with pure water, extract wit...
Embodiment 2
[0022]Weigh 1.37g (3mmol) of purchased ursolic acid (99%) and add it to a 100mL round-bottomed flask, add 50mL of dichloromethane, stir well until completely dissolved, add 1.61g (about 7.5mmol) of Sarrite reagent, and heat at 40°C Stir the reaction for 1 h, add pure water to quench the reaction, separate the layers in a separatory funnel, extract the aqueous layer once more with dichloromethane, combine the dichloromethane extracts, and pass through a silica gel chromatography column (dichloromethane:ethyl acetate, 5: 1) separation and purification to obtain ursolic acid C 3 Oxidation product of hydroxyl group (UA-O) 1.02g, purity 98.6%.
[0023] Take 0.28g UA-O (0.60mmol) into a 100mL three-necked flask, add ammonia-saturated absolute ethanol solution to fully dissolve, add 1.0mL isopropyl titanate solution under the protection of argon, stir and react at 35°C for 5h, then drop to to room temperature, then add 0.13g (0.6mmol) sodium triacetoxyborohydride to react for 2h, th...
Embodiment 3
[0028] Weigh 1.37g (3mmol) of ursolic acid (98.6%) into a 100mL round-bottomed flask, add 50mL of dichloromethane, stir well until completely dissolved, add 1.61g (about 7.5mmol) of Sarrite reagent, and stir at 15°C for reaction 6h, add pure water to quench the reaction, separate the liquid in a separatory funnel, extract the aqueous layer once more with dichloromethane, combine the dichloromethane extracts, and pass through a silica gel chromatography column (dichloromethane:ethyl acetate, 5:1) Separation and purification to obtain ursolic acid C 3 Oxidation product of hydroxyl group (UA-O) 1.19g, purity 98.7%.
[0029] Take 0.28g UA-O (0.60mmol) into a 100mL three-necked flask, add ammonia-saturated absolute ethanol solution to dissolve, add 1.0mL isopropyl titanate solution under the protection of argon, stir and react at 35°C for 5h, then drop to At room temperature, add 0.13g (0.6mmol) sodium triacetoxyborohydride and react for 3h, then quench the reaction with pure wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com