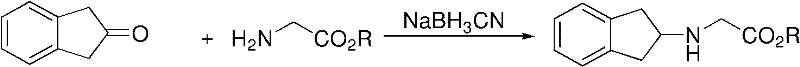Preparation method of N-(2- indanyl) amino acid alkyl ester
A technology of glycine alkyl ester and ethyl acetate, which is applied in the field of N-amino acid alkyl ester preparation, can solve problems such as low yield, environmental pollution by by-products, etc., and achieve the effect of avoiding toxic substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Mix 5.27g glycine methyl ester hydrochloride (0.042mol), 9.62g sodium triacetoxyborohydride (0.046mol), 2.17ml glacial acetic acid (0.038mol), 90ml 1,2-dichloroethane, stir and mix Evenly, cool in an ice bath to 5°C;
[0033] 2) Add 5 g of 2-indanone (0.038 mol) dissolved in 10 ml of 1,2-dichloroethane dropwise, about 25 minutes after the drop. React at 10°C for 40min (until no bubbles are generated in the system), transfer to room temperature and react at 30°C for 3h, and detect the end point by TLC.
[0034] 3) After the reaction, the solvent was removed under reduced pressure, 50ml of water was added under stirring, and 20% H 3 PO 4 50ml was adjusted to pH=2-3.
[0035] 4) Extract with toluene four times (25×4ml), under cooling, slowly add sodium carbonate to the water layer while stirring to adjust the pH to 7-8, extract with chloroform, anhydrous Na 2 SO 4 Dry, remove the solvent under reduced pressure to obtain the extract;
[0036] 5) This material was ...
Embodiment 2
[0038] 1) Mix 13.95g tert-butyl glycine hydrochloride (0.083mol), 4.33ml glacial acetic acid (0.076mol), 19.24g sodium triacetoxy borohydride (0.0908mol), 200ml of 1,2-dichloroethane , cooled to 5°C in an ice bath;
[0039] 2) 10g (0.076mol) 2-indanone 30ml, 1,2-dichloroethane solution was added dropwise under stirring, reacted at 10°C for 1h, transferred to room temperature at 30°C for 1h, and detected the end point by TLC.
[0040] 3) After the reaction is completed, the solvent is removed under reduced pressure, and 100ml of water is added under stirring, and 20% H 3 PO 4 100ml was adjusted to pH=2-3.
[0041] 4) Extract four times with toluene (50×4ml). Under cooling, slowly add sodium carbonate to the water layer while stirring to adjust the pH to 7-8. During the addition of sodium carbonate, a precipitate precipitates, vacuum-filter, and vacuum-dry After obtaining a solid;
[0042] 5) Recrystallized with ethanol / water (3 / 1) to obtain 14.11 g of white crystals, the y...
Embodiment 3
[0044] 1) Mix 13.95g tert-butyl glycine hydrochloride (0.083mol), 19.24g sodium triacetoxyborohydride (0.0908mol), 200ml 1,2-dichloroethane, and cool to 5°C in an ice bath;
[0045] 2) Add 10 g (0.076 mol) of 2-indanone in 30 ml of 1,2-dichloroethane dropwise under stirring, react at 10°C for 1 hour, turn to room temperature and react at 30°C for 1 hour, and check the end point by TLC.
[0046] 3) After the reaction is completed, the solvent is removed under reduced pressure, and 100ml of water is added under stirring, and 20% H 3 P0 4 100ml was adjusted to pH=2-3.
[0047] 4) Extract four times with toluene (50×4ml). Under cooling, slowly add sodium carbonate to the water layer while stirring to adjust the pH to 7-8. During the addition of sodium carbonate, a precipitate precipitates, vacuum-filter, and vacuum-dry After obtaining a solid;
[0048] 5) Recrystallized with ethanol / water (3 / 1) to obtain 13.69 g of white crystals, the yield of N-(2-indanyl)glycine tert-butyl e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com