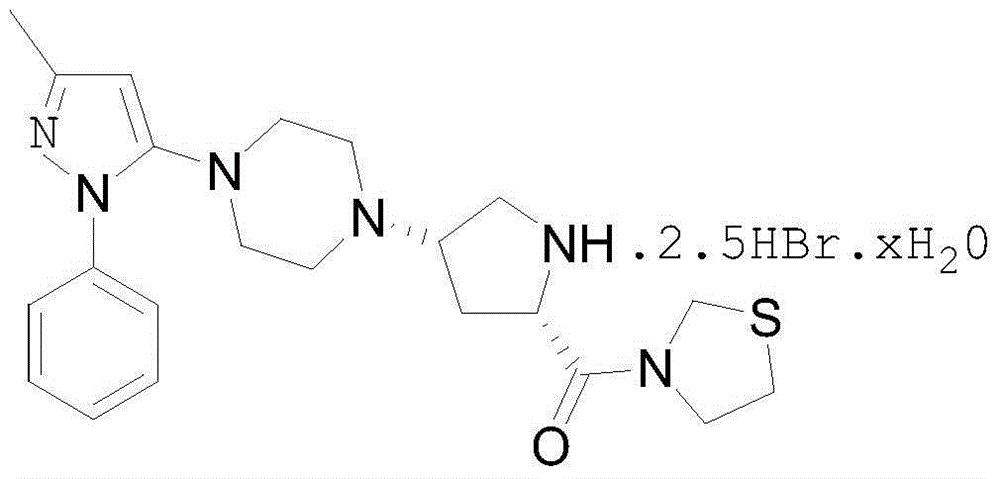
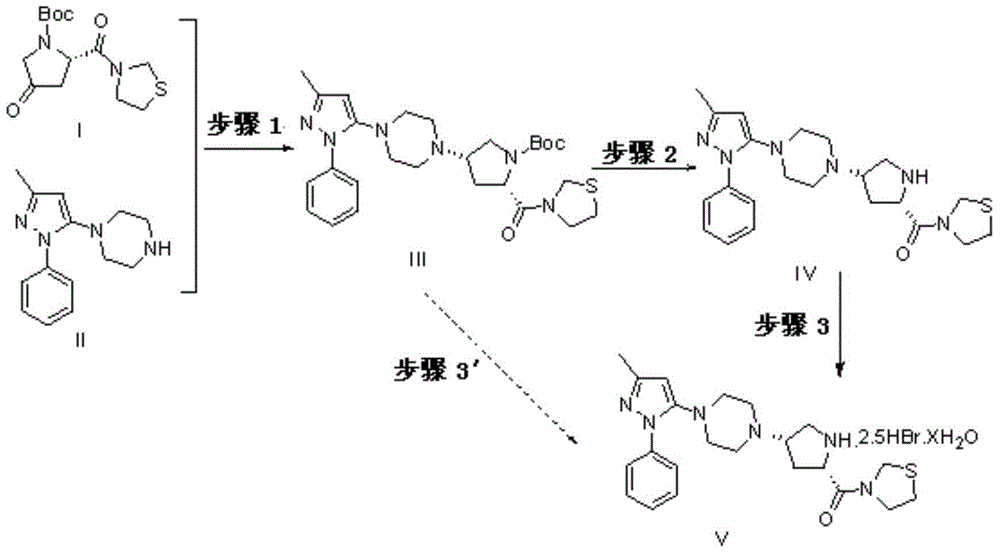
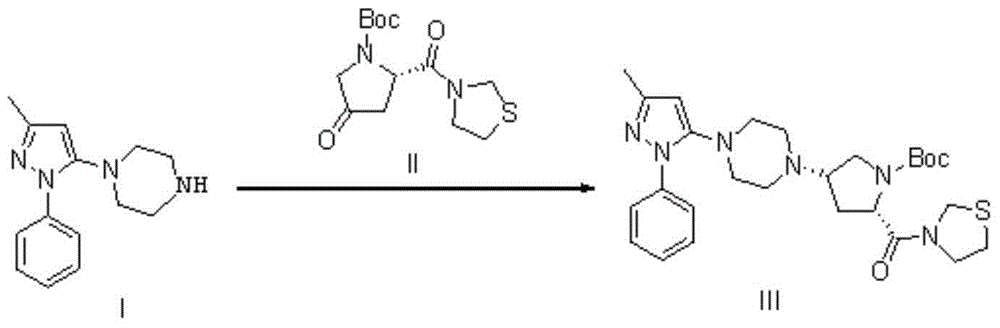
Teneligliptin synthesis method
A synthetic method, the technology of tiagliptin, applied in the field of drug synthesis, can solve the problems of hydrobromic acid being volatile, many by-products after stirring, difficult to complete the reaction of raw materials, etc., to achieve increased solubility and reducibility, high total yield, The effect that is conducive to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0032] Experimental example 1 toluene and tetrahydrofuran volume ratio screening experiment
[0033] Method 1 Method 1 of the prior art, add 20L toluene to the reactor, and 808g formula (I) (1-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)] piperazine), 1000g formula (II) (3-[(2S)-1-(1,1-dimethylacetylcarbonyl)-4-oxopyrrolidin-2-ylcarbonyl]thiazolidine), 200g acetic acid Add it into the reaction kettle, dissolve it with the treated toluene and tetrahydrofuran mixed solution, add 1413g sodium triacetoxyborohydride, react at 20°C for 2h, concentrate the solvent at 40°C, add 10L ethyl acetate, and use 30L saturated ammonium chloride After washing twice, and drying the organic layer with anhydrous sodium sulfate for 0.5 h, the yield was 87%, and the product purity was 88.17%.
[0034] Method 2 (step A of Example 1): Add 10L of toluene, 10L of tetrahydrofuran and 5g of benzophenone into the reaction kettle, then add 100g of sodium pellets, heat to boiling at 70°C until the solution turns ...
Embodiment 1
[0043]A. Add 10L of toluene, 10L of tetrahydrofuran and 5g of benzophenone into the reaction kettle, then add 100g of sodium pellets, heat to boiling at 70°C until the solution turns blue, and distill off the solvent for later use. With 808g formula (I) (1-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)] piperazine), 1000g formula (II) (3-[(2S)- 1-(1,1-Dimethylacetylcarbonyl)-4-oxopyrrolidin-2-ylcarbonyl]thiazolidine), 200g of acetic acid were added to the reaction kettle, and dissolved in a mixed solution of 10L of toluene and 10L of tetrahydrofuran , add 1413g of sodium triacetoxyborohydride, react at 20°C for 2h, concentrate the solvent at 40°C, add 10L of ethyl acetate, wash twice with 30L of saturated ammonium chloride, dry the organic layer with anhydrous sodium sulfate for 0.5h, reduce Concentrate under reduced pressure to 1710 g of light yellow solid (formula III), the yield is 98%, and the product purity is 98.43%.
[0044] B. Dissolve 1700g of the intermediate (formula III) ...
Embodiment 2
[0047] A. Add 13.5L of toluene, 6.5L of tetrahydrofuran and 3g of benzophenone into the reaction kettle, then add 80g of sodium pellets, heat to boiling at 70°C until the solution turns blue, and distill off the solvent for later use. With 808g formula (I) (1-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)] piperazine), 1000g formula (II) (3-[(2S)- 1-(1,1-Dimethylacetylcarbonyl)-4-oxopyrrolidin-2-ylcarbonyl]thiazolidine), 200g of acetic acid were added to the reaction kettle, dissolved with the treated toluene and tetrahydrofuran mixed solution, and added 1413g of sodium triacetoxyborohydride, reacted at 20°C for 2h, concentrated the solvent at 40°C, added 10L of ethyl acetate, washed twice with 30L of saturated ammonium sulfate, dried the organic layer with anhydrous sodium sulfate for 0.5h, and concentrated under reduced pressure to The light yellow solid (formula III) was 1668g, the yield was 95%, and the product purity was 90.43%.
[0048] B. Dissolve 1660g of the intermediate (fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com