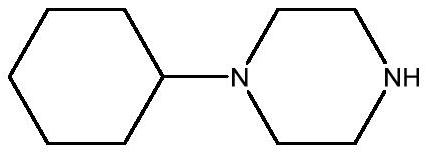
Preparation method of 1-cyclohexylpiperazine
A technology of cyclohexylpiperazine and cyclohexyl is applied in the field of synthesis of small molecule drug intermediates, and can solve problems such as troublesome post-processing, complicated steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
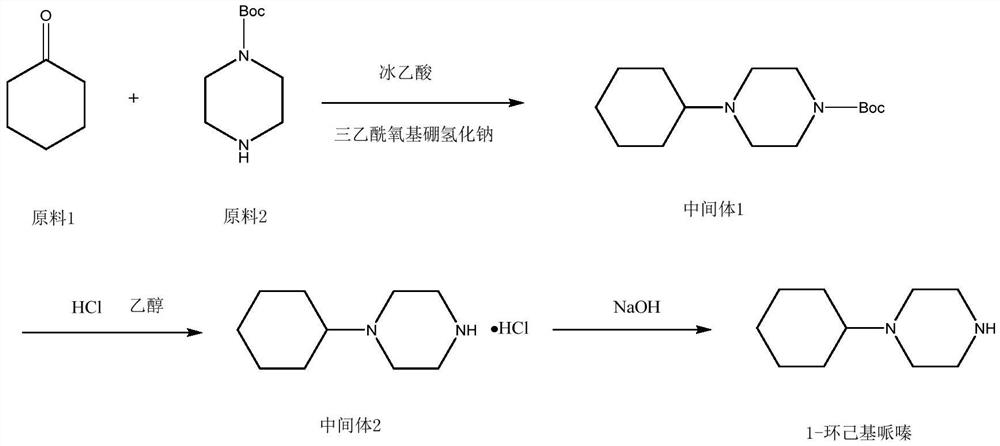
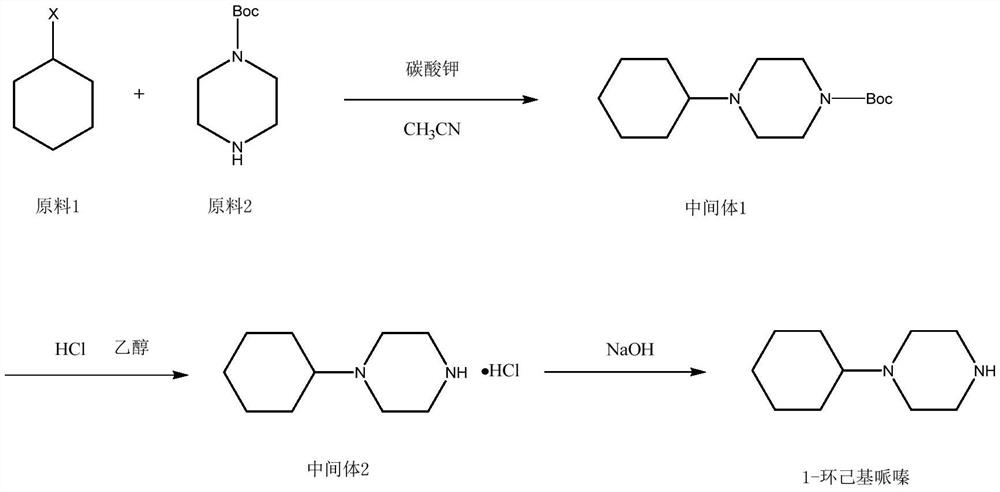
Embodiment 1
[0026]
[0027] Add 4.8kg of cyclohexyl bromide (29.5mol) into a 50L reactor, add anhydrous acetonitrile 30kg, 1-Boc-piperazine 5kg (26.84mol), and 4.08kg (29.5mol) of potassium carbonate under stirring. Heat up to react, reflux for 2 hours, TLC monitors the reaction process, after the reaction is completed, cool to room temperature, filter, and the filtrate is concentrated to dryness to obtain 7.0 kg of intermediate 1 red oil. The purity of the product as determined by GC is 98.5%. The rate is 96.6%.
Embodiment 2
[0028] Embodiment 2: the preparation of intermediate 2 cyclohexylpiperazine hydrochloride
[0029]
[0030] At room temperature, put 7kg of intermediate 1 obtained in the previous step into a 50L reactor, add 26kg of absolute ethanol and 6.6L of concentrated hydrochloric acid, pay attention to controlling the speed when adding concentrated hydrochloric acid, the reaction will generate a lot of bubbles and exotherm, pay attention to safety, After adding the hydrochloric acid, gradually heat up to the reflux temperature, and control the temperature rise rate not to be too fast. If the temperature rise rate is too fast, the reaction will be uncontrollable and dangerous. After the reflux reaction for about 4 hours, monitor the reaction process by TLC. After the reaction is completed, distill under reduced pressure. Evaporate the solvent to dryness, then cool down and add 8kg of isopropanol for beating, cool down to room temperature and continue to stir for 2 hours. After fully s...
Embodiment 3
[0031] Embodiment 3: Preparation of 1-cyclohexylpiperazine
[0032]
[0033] Add the product from the previous step into a 20L reaction kettle, add 6kg of pure water to stir and dissolve, adjust the pH to 14 with 20% sodium hydroxide solution, and add 6kg of dichloromethane to extract at the same time, and extract the water layer with dichloromethane after liquid separation 2 times, each 2kg, and keep the pH of the water layer is 14, merge the dichloromethane layer, dry over anhydrous sodium sulfate, after filtering, evaporate to dryness under reduced pressure, obtain semi-solid and semi-oily product after evaporation to dryness, then the product Distillation under reduced pressure gave 3.1 kg of an oil with a melting point of 34°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com