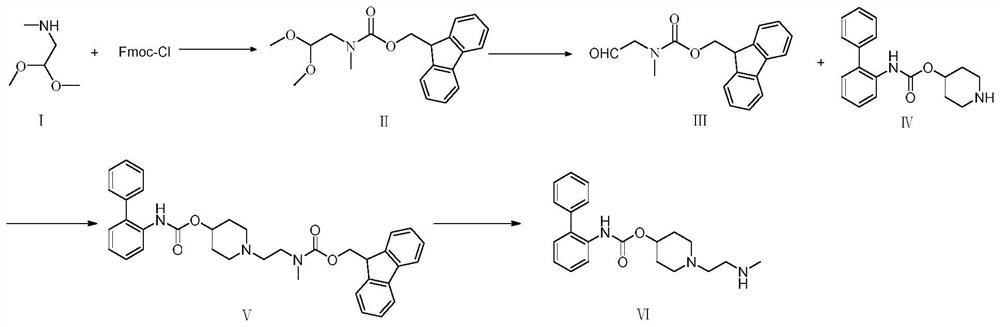
Preparation method of rafenasin intermediate
A technology for intermediates and compounds, applied in the field of chemical pharmacy, achieves the effects of less environmental pollution, low production cost, mild and safe reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
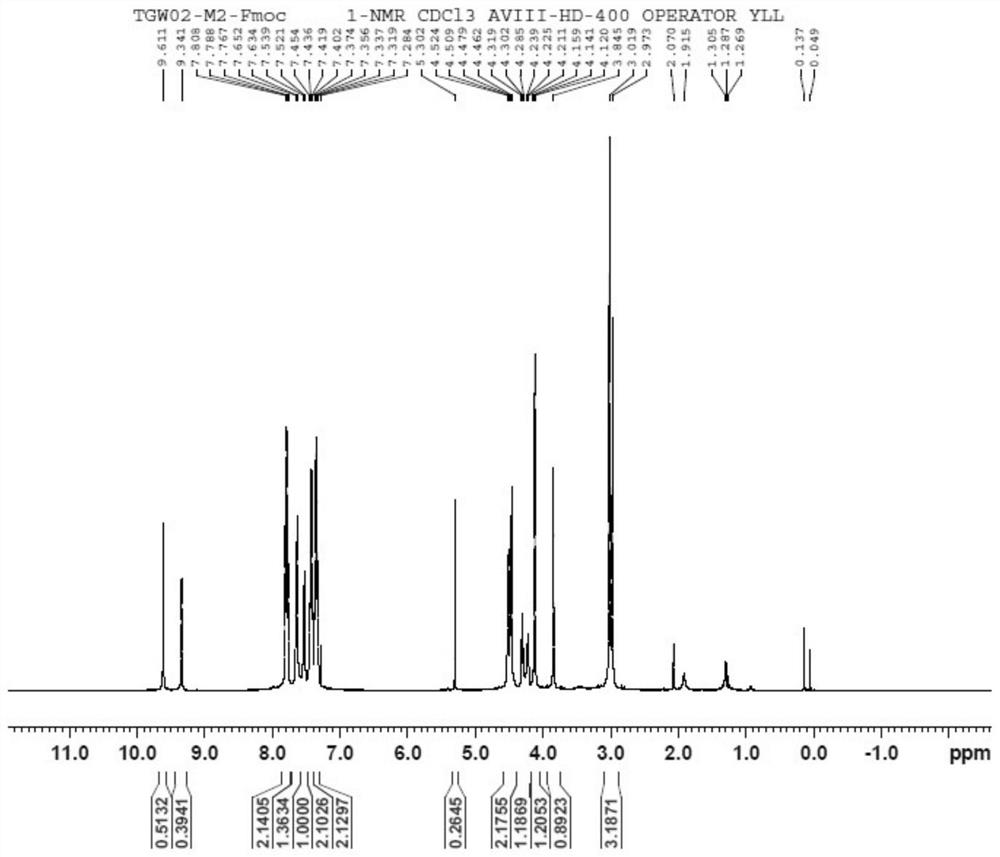
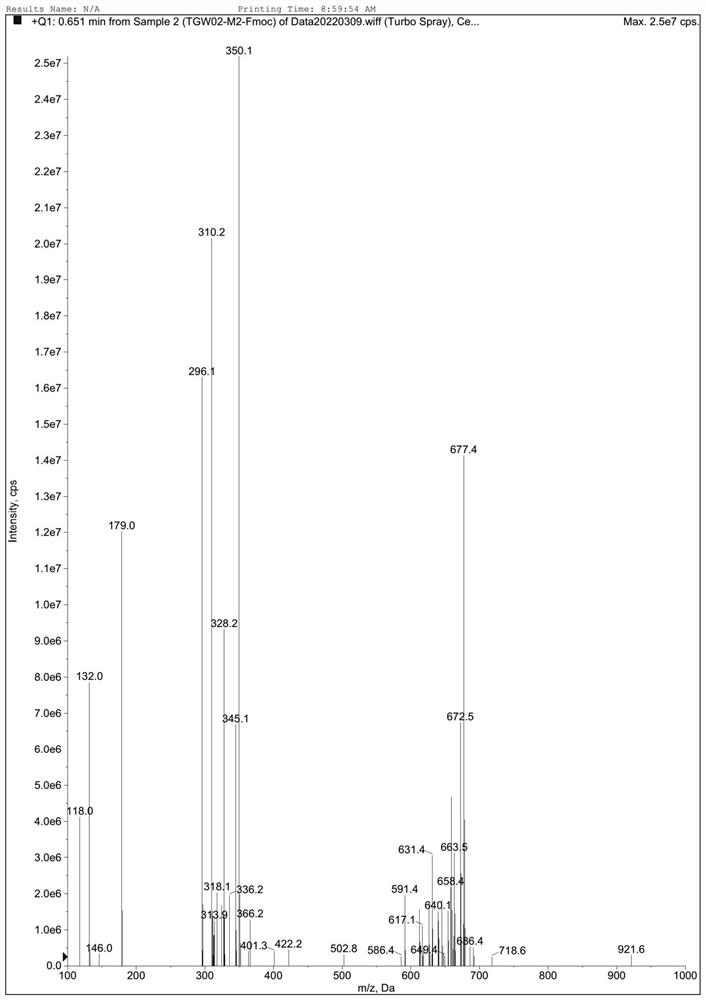
[0068] Embodiment 1: the synthesis of refenacin intermediate ( figure 1 )
[0069] (1) Add 76.32g of sodium carbonate to a 2L reaction flask, stir to dissolve with 600mL of water, cool down to 0°C; weigh 82.5g of methylaminoacetaldehyde dimethyl acetal material and add 600mL of 2-methyltetrahydrofuran Make a mixed solution, add it to the reaction bottle, and keep it warm at 0°C; weigh a total of 102.3g of Fmoc-Cl, add it dropwise to the reaction bottle, and keep it warm at 0°C during the dropping process; after 1h of dropping, raise the temperature to 20°C and keep it warm for 5h. Stand for separation and keep the organic phase to obtain formula II. The organic phase is directly used for feeding in the next step.
[0070] (2) The organic phase in step (1) was reacted with 600 mL of 3mol / L hydrochloric acid aqueous solution at 20° C. for 8 h; layer; the aqueous phases were combined, 600 mL of EA was added to stir and extract, and the layers were allowed to stand; the organic ...
Embodiment 2
[0074] The synthetic route of the refenacin intermediate in the present invention has been fully optimized for parameters, and the parameter optimization results are as follows:
[0075] 1. Fmoc protection reaction of amino group
[0076] (1) The Fmoc protection reaction solvent type optimization result of amino group is as follows (table 1), and other parameters are with step (1) in embodiment 1:
[0077] Table 1
[0078]
[0079] From the above results, it can be seen that when 2-methyltetrahydrofuran is used as the Fmoc protection reaction solvent of the amino group, Fmoc-Cl can react completely, while the other three solvents Fmoc-Cl are all remaining.
[0080] (2) The Fmoc protection reaction solvent ratio optimization result of amino group is as follows (table 2), and other parameters are with step (1) in embodiment 1:
[0081] Table 2
[0082]
[0083] From the above results, it can be seen that when the volume ratio of 2-methyltetrahydrofuran is 6:1, Fmoc-Cl c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com