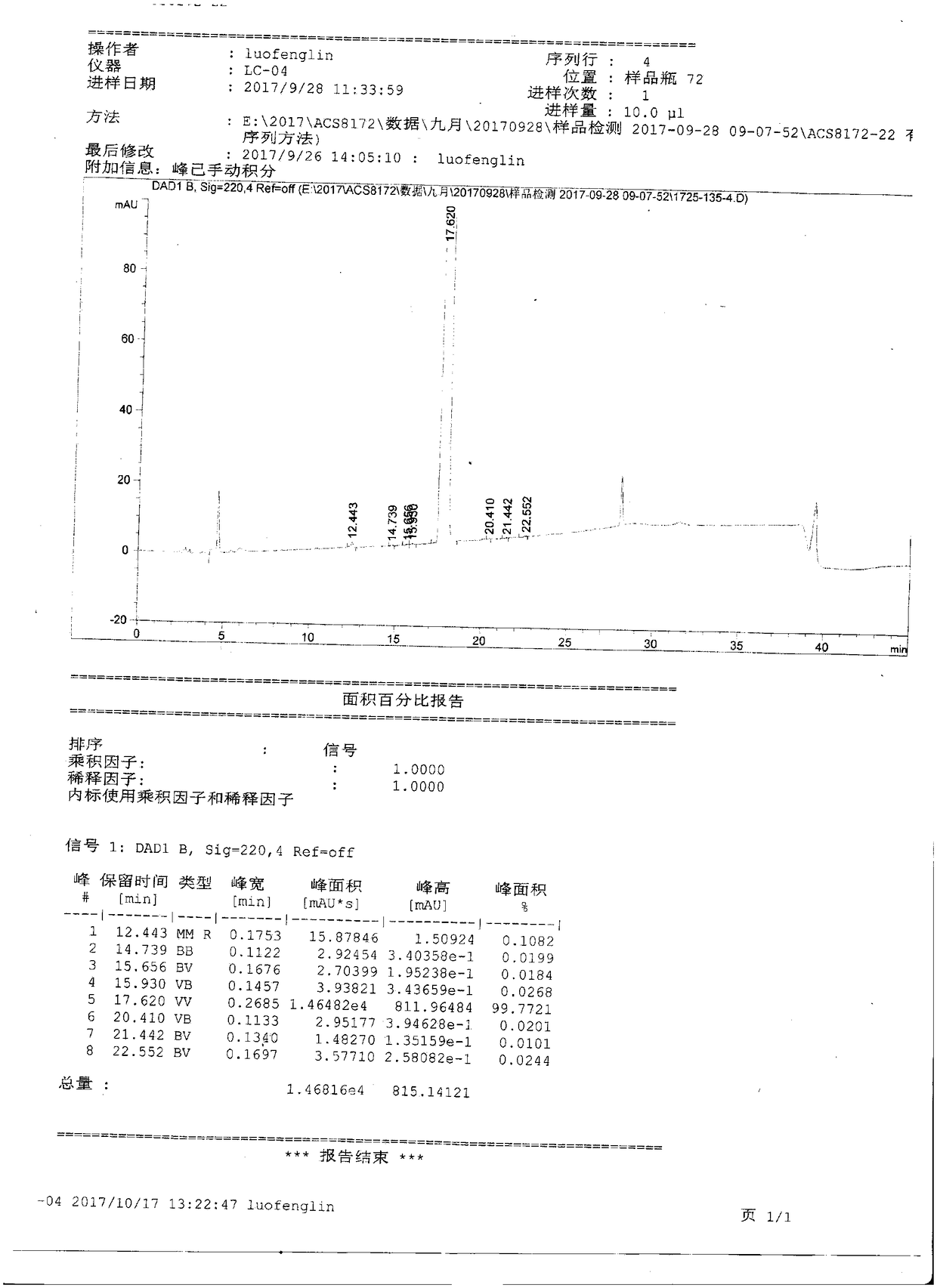
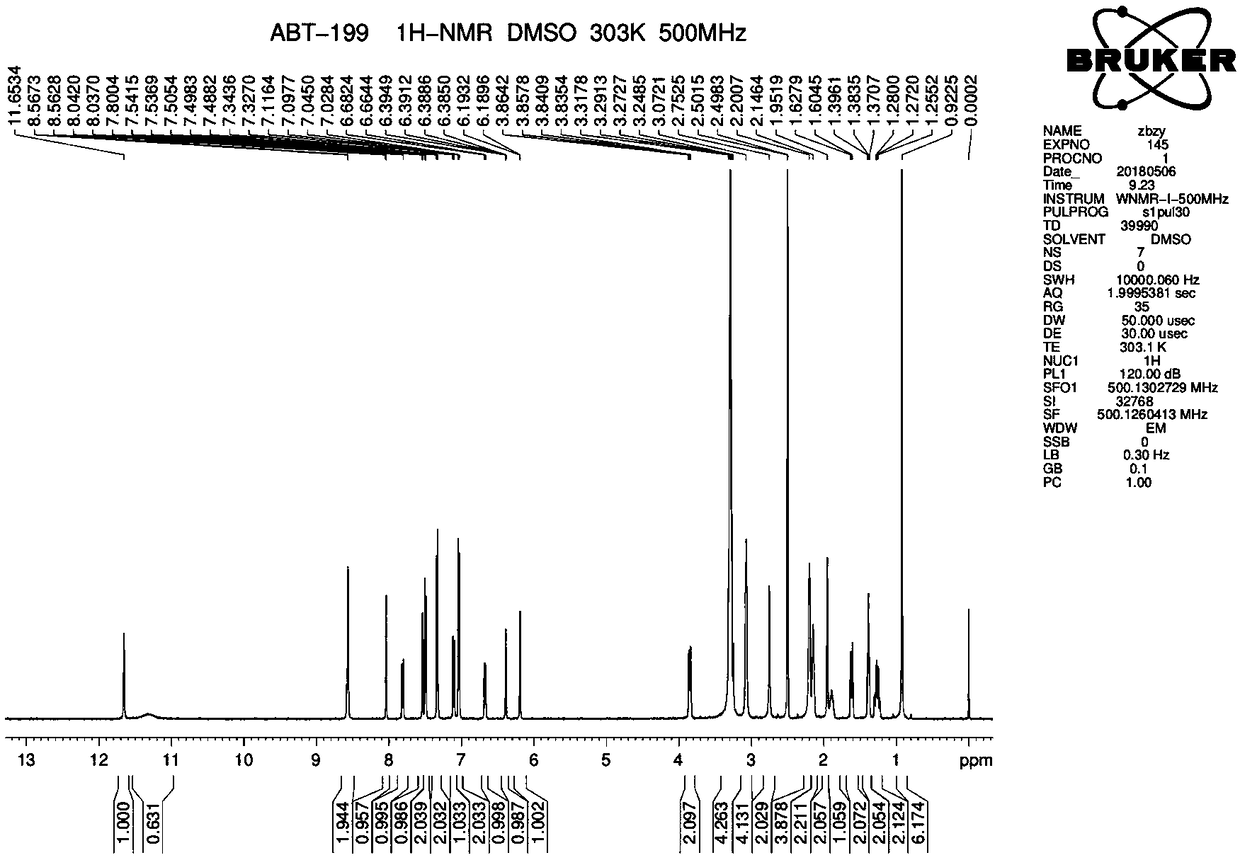
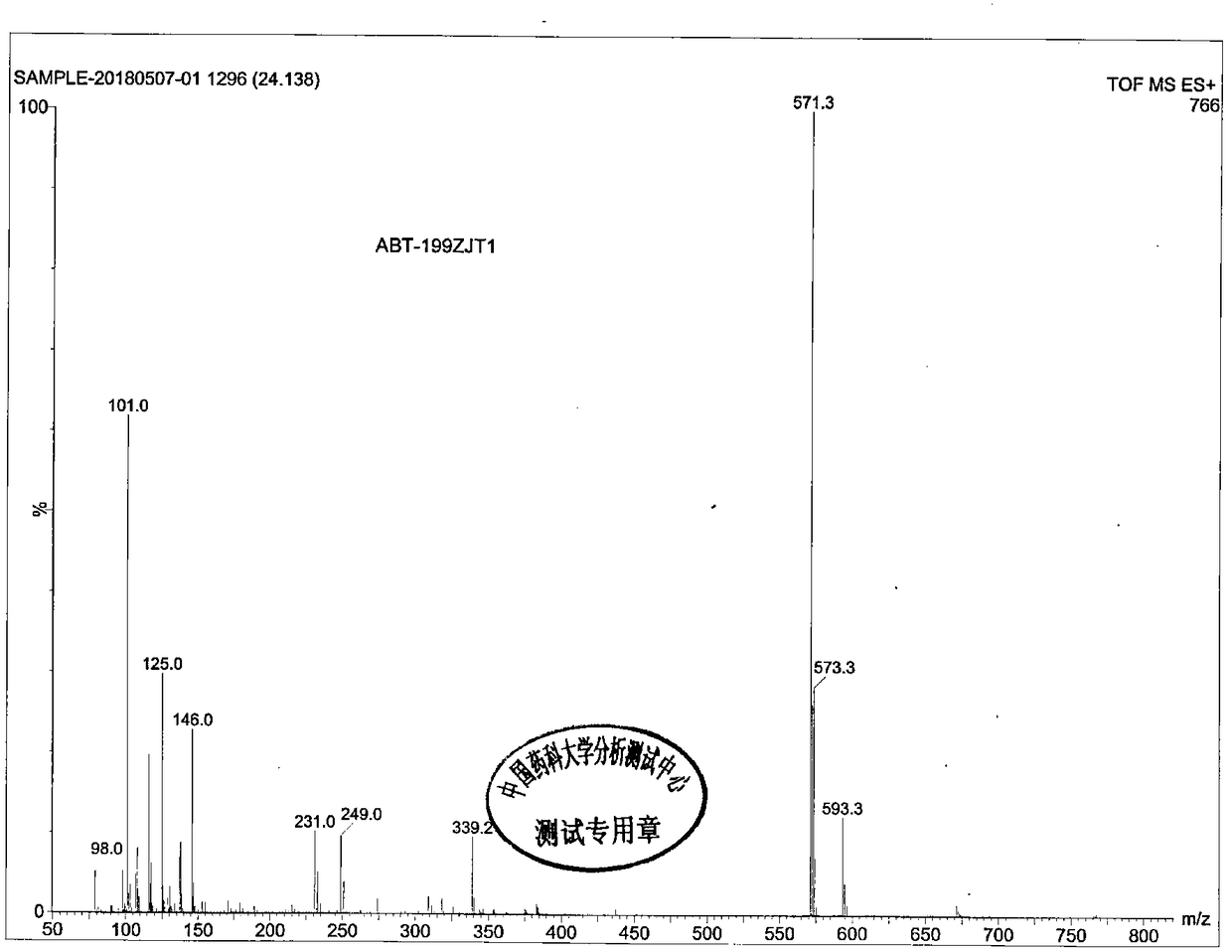
Preparation method of B cell lymphoma factor-2 inhibitor ABT-199
A technique for ABT-199, lymphoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of Compound IV
[0057] Add 34.2g (0.20mol) compound II, 3.1g (8.4mmol) tetra-tert-butyl ammonium iodide (TBAI), 30.4g (0.22mol) potassium carbonate and 41.0g (0.22mol) compound III and 350mLDMSO in 1L reaction flask . After the addition, the system was heated to 100°C to react for about 5 hours. After cooling down to room temperature, the reaction was continued for 10 hours. Controlled by HPLC or TLC, the reaction of the raw materials was complete, and the reaction was stopped. After filtration, 300 mL of ethyl acetate and 83 mL of concentrated hydrochloric acid solution were added to the filtrate, stirred at room temperature for 1 h, and filtered to obtain 47.0 g of crude compound IV as a pale yellow solid. Directly used in the next step, HPLC: 97.8%, yield: 86.3%.
[0058] Synthesis of Compound V
[0059] Add 33.4g (0.46mol) of anhydrous DMF and 80mL of dichloromethane into a 500mL reaction flask, cool the system down to -5°C, and add 64.7g (0.42mol) of ...
Embodiment 2
[0068] Synthesis of Compound IV
[0069] Add 34.2g (0.20mol) compound II, 3.4g (10.5mmol) tetra-tert-butylammonium bromide (TBAB), 30.4g (0.22mol) potassium carbonate and 41.0g (0.22mol) compound III and 500mLTHF in 1L reaction flask . After the addition is complete, the system is heated to 30°C for about 5 hours, and the reaction is continued for 12 hours after cooling down to room temperature. The reaction is stopped when the reaction is completed by HPLC or TLC. After filtering, HCl gas was passed into the filtrate under stirring at room temperature until no solid was precipitated in the system. Filtration gave 44.2 g of the crude product of compound IV as a pale yellow solid. Directly used in the next step, HPLC: 97.8%, yield: 81.2%.
[0070] The synthesis of compound V is the same as in Example 1.
[0071] Synthesis of Compound VI
[0072] 30.0 g (0.12 mol) of compound V and 300 mL of xylene solution were added to a 2L reaction flask, and 40.8 g (0.15 mol) of compoun...
Embodiment 3
[0077] Synthesis of Compound IV
[0078] Add 34.2g (0.20mol) compound II, 2.9g (10.5mmol) tetra-tert-butyl ammonium chloride (TBAC), 30.4g (0.22mol) potassium carbonate and 41.0g (0.22mol) compound III and 500mL toluene. After the addition, the system was heated up to 80°C for about 5 hours, and continued to react for 10 hours after cooling down to room temperature. Controlled by HPLC or TLC, the reaction of the raw materials was complete, and the reaction was stopped. Filter, and the filtrate is spin-dried under reduced pressure. Add 200 mL of tetrahydrofuran into the system, and feed HBr gas into the filtrate with stirring at room temperature until no solid is precipitated in the system. After filtration, 49.2 g of crude yellow solid of compound IV was obtained. Directly used in the next step, HPLC: 97.6%, yield: 77.9%. The synthesis of compound V is the same as in Example 1.
[0079] Synthesis of Compound VI
[0080] 30.0 g (0.12 mol) of compound V and 300 mL of 1,4-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com