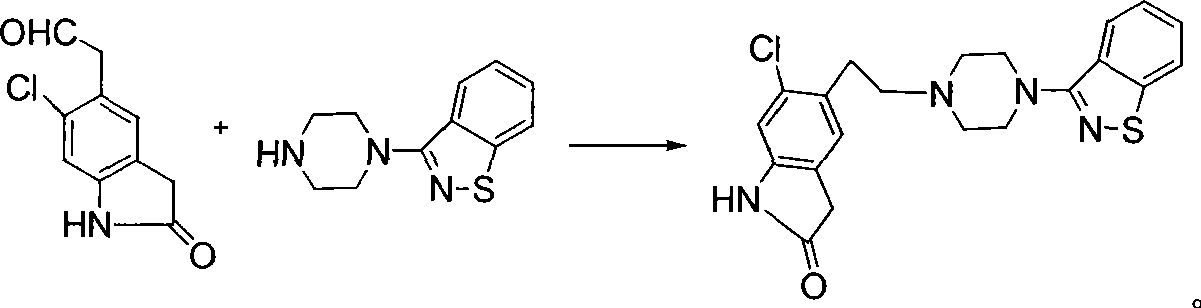
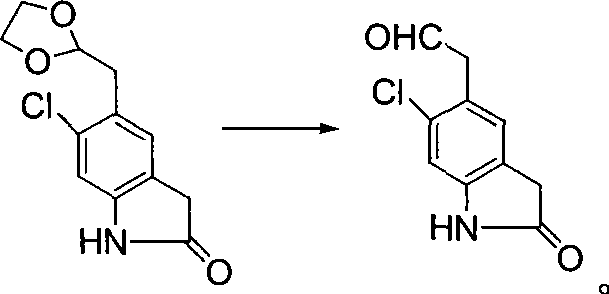
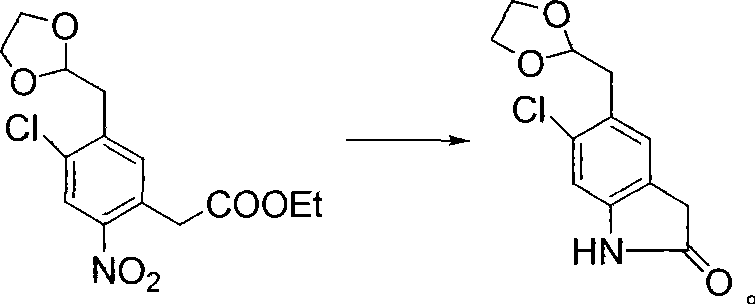
Synthetic method of ziprasidone
A technology of ziprasidone and dioxolane, applied in the field of drug synthesis, can solve the problems of low reaction yield and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation and synthesis of 2,5-dichloro-4-nitrotoluene
[0065]
[0066] Add 100 g of 2,5-dichlorotoluene and 50 ml of concentrated sulfuric acid to a 500 ml flask equipped with a thermometer with mechanical stirring and a condenser, and start stirring to cool to 10°C. Measure 80ml of concentrated sulfuric acid in another beaker, weigh fuming nitric acid 43.2g (95%), add dropwise in the beaker, and pay attention to cooling. Add the mixed acid into a 100ml dropping funnel and then slowly drop it into a 500ml flask. At the beginning, control the temperature at 10-15°C, and slowly rise to about 25°C when the solid precipitates. Keep adding at this temperature. After the addition, continue to stir at 35°C for 2 hours, then pour into ice water to freeze. The precipitated solid was suction filtered, and the obtained solid was recrystallized with 150 ml of ethanol. The product (80 g, yield 63%) was obtained. 1 H NMR (400MHz, CDCl 3 )δ: 2.44(s, 3H, CH 3 ),...
Embodiment 2
[0067] Example 2: Preparation of 2-(2,5-dichloro-4-nitrobenzene)-N,N-dimethylethyleneamine
[0068]
[0069] In a 300ml flask equipped with a thermometer with mechanical stirring and condenser, add 2,5-dichloro-4-nitrotoluene 33.8g, dimethoxy-N,N-dimethylmethylamine (29.3g , 1.5eq), DMF70ml, after the addition, the stirring and reflux temperature was maintained at about 120 degrees, and the reaction was carried out for 4 hours. Stop heating, cool to 100°C, pour the reaction solution into 150ml of water for washing, and a purple-brown solid precipitates out. The filtered cake was washed with water and pressed dry. The solids were oven dried at 90 degrees. The product (40 g, yield 93%) was obtained. 1 H NMR (400MHz, CDCl 3 )δ: 3.00 (s, 6H, 2NCH 3 ), 5.29(d, J=13.2Hz, 1H, CH=), 7.07(d, J=13.2Hz, 1H, NCH=), 7.34(s, 1H, ArH), 8.03(s, 1H, ArH); 13 C NMR (100MHz, d-DMSO) δ: 42.1, 42.6, 88.3, 122.2, 124.5, 125.6, 127.5, 138.0, 145.3, 148.5; Ms: M=260, Found (260, M + ), (2...
Embodiment 3
[0070] Example 3: Preparation of 2-(2,5-dichloro-4-nitrobenzyl)-1,3-dioxolane
[0071]
[0072] Add 2-(2,5-dichloro-4-nitrobenzene)-N,N-dimethylethyleneamine (40g), toluene 80ml successively in a 300ml flask equipped with a thermometer with mechanical stirring and condenser Oxalic acid (38.6g, 2eq), ethylene glycol 38g. Stir and reflux for 8 hours after addition. After the reaction was completed, the reaction liquid was added to 100 ml of water to wash, and then extracted with 200 ml of ethyl acetate, and the organic layer was collected, and the organic layer was washed with 100 ml of water. The organic layer was dried over anhydrous magnesium sulfate. The solvent was evaporated, and the residue was recrystallized with a mixed solvent of 50ml ethyl acetate and 100ml of n-hexane to obtain the product (22g, yield 52%). 1 H NMR (400MHz, CDCl 3 )δ 3.16 (d, J=4.6Hz, 2H, CH 2 ), 3.86-3.90 (m, 2H, OCH 2 ), 3.95-3.99 (m, 2H, OCH 2 ), 5.15(t, J=4.6Hz, 1H, CH), 7.58(s, 1H, ArH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com