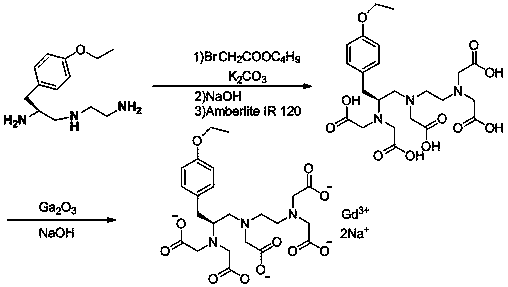
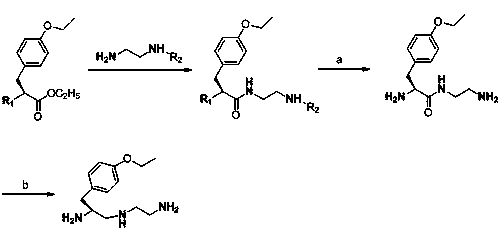
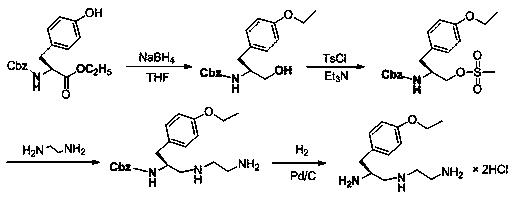
Synthesis method of (S)-1-(4-ethyoxyl benzyl)-3-azapentane-1,5-diamine trihydrochloride
A technology of diamine trihydrochloride and ethoxybenzyl group is applied in the field of pharmaceutical chemical synthesis, which can solve the problems of non-production process, high environmental protection pressure and high equipment requirements, and achieves novel and concise steps, convenient post-processing, and mild reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take a 100 mL two-neck reaction flask, N 2 Add compound 1 sequentially under protection ( R )-2-(tert-butoxycarbonyl)amino)-3-(4-ethoxyphenyl)propanoic acid methyl ester 3.00 g (9.27 mmol), 20 mL of anhydrous toluene, placed in -40 ℃ cold trap Stir for later use; take another 100 mL cock reaction bottle, N 2 Add 70wt% red aluminum toluene solution (dihydrobis(2-methoxyethoxy)sodium aluminate 5.35 g (18.5 mmol)) under protection, then add morpholine (1.78 g , 20.4 mmol), continue to stir for 2 h after the dropwise addition; then slowly add the red aluminum solution dropwise to the first reaction flask, N 2 Under protection, react at -40 °C for 3 h. Add NaSO after the reaction is complete 4 10H 2 O (5.09 g, 18.5 mmol) was used to quench the reaction, then 20 mL of ethyl acetate was added to dilute, suction filtered and concentrated by rotary evaporation, and 1.39 g of white solid was obtained by column chromatography (petroleum ether: ethyl acetate = 10:1). Tha...
Embodiment 2
[0034] Take a 100 mL two-neck reaction flask, N 2 Add compound 1 sequentially under protection ( R )-2-(tert-butoxycarbonyl)amino)-3-(4-ethoxyphenyl)propionic acid methyl ester, 3.00 g (9.27 mmol), 20 mL of anhydrous toluene, placed in a -40 ℃ cold trap Stir for later use; take another 100 mL cock reaction bottle, N 2 Add 70% red aluminum toluene solution (sodium dihydrobis(2-methoxyethoxy)aluminate, 16.1 g, 55.5 mmol) under protection, and then add morpholine (5.34 g , 61.2 mmol), continue to stir for 2 h after the dropwise addition; then slowly add the red aluminum solution dropwise to the first reaction flask, N 2 Under protection, react at -40 °C for 3 h. Add NaSO after the reaction is complete 4 10H 2 O (15.3 g, 55.5 mmol) was used to quench the reaction, then 20 mL of ethyl acetate was added to dilute, suction filtered and concentrated by rotary evaporation, and 1.71 g of white solid was obtained by column chromatography (petroleum ether: ethyl acetate = 10:1). Na...
Embodiment 3
[0038] Take a 100 mL two-neck reaction flask, N 2 Add compound 1 sequentially under protection ( R )-2-(tert-butoxycarbonyl)amino)-3-(4-ethoxyphenyl)propionic acid methyl ester, 3.00 g (9.27 mmol), 20 mL of anhydrous toluene, placed in a -40 ℃ cold trap Stir for later use; take another 100 mL cock reaction bottle, N 2 Add 70% red aluminum toluene solution (sodium dihydrobis(2-methoxyethoxy)aluminate, 16.1 g, 55.5 mmol) under protection, and then add morpholine (5.34 g , 61.2 mmol), continue to stir for 2 h after the dropwise addition; then slowly add the red aluminum solution dropwise to the first reaction flask, N 2 Under protection, react at -55 °C for 2 h. Add NaSO after the reaction is complete 4 10H 2 O (15.3 g, 55.5 mmol) was used to quench the reaction, then 20 mL of ethyl acetate was added to dilute, suction filtered and concentrated by rotary evaporation, and 2.08 g of a white solid was obtained by column chromatography (petroleum ether: ethyl acetate = 10:1). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com