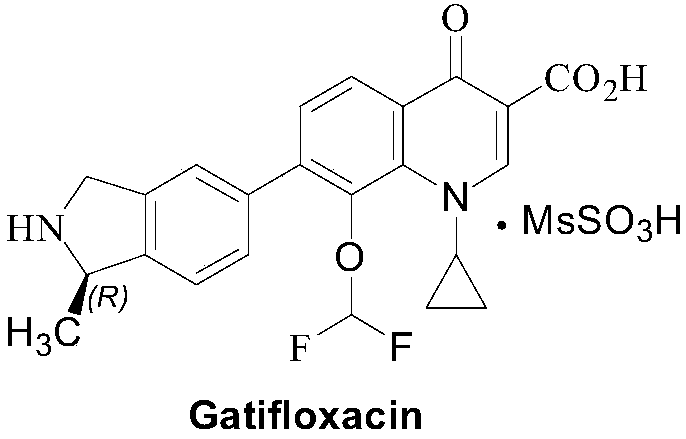
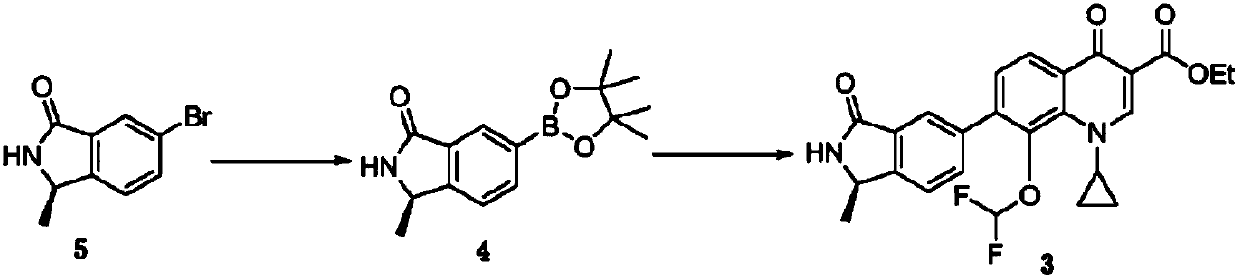
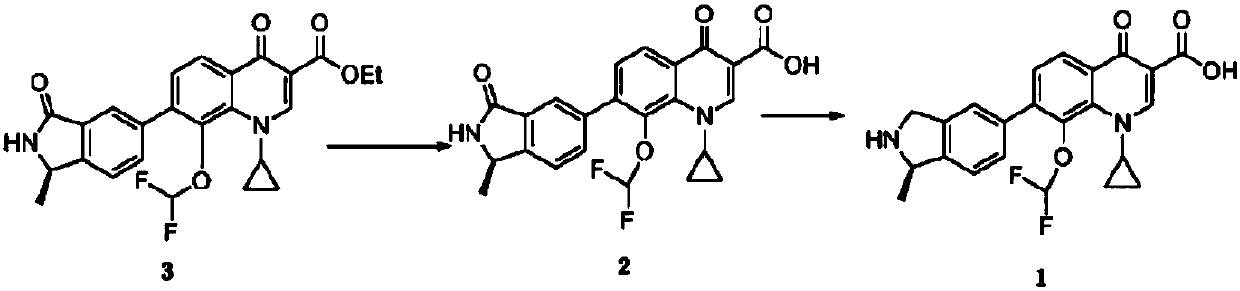
A kind of (r)‑n‑boc‑5‑bromo‑1‑methylisoindoline and its preparation method and application
A technology of methylisoindoline and -n-boc-5-, which is applied in the field of drug synthesis, can solve problems that no one has raised, and achieve the effects of novel and unique steps, high practical application value, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) Preparation of 4-bromo-phenethylamine
[0057]
[0058] Add 1000g of p-bromoacetophenone and 1445g of ammonium formate into a 5L reaction flask, and slowly heat to 120°C. The two raw materials slowly dissolve and become liquid. Start stirring and keep at 120°C for 2 hours. Then the reaction temperature was raised to 180° C. and kept at this temperature for 5 hours. Stop the reaction, after cooling, add water, dichloromethane, separate layers, concentrate the organic phase, add 670 ml of concentrated hydrochloric acid and 1000 ml of water, reflux, 1-2 hours, the reactant first has solids, then clarifies, and then a large amount of solids are precipitated , the reaction is over, after cooling slightly, add 1000 milliliters of toluene, and cool to room temperature under stirring. Filtration, the solid was fully washed twice with toluene, and the solid was dried to obtain the hydrochloride salt of the product, which was neutralized with sodium hydroxide, extracted w...
Embodiment 2
[0070] (1) Preparation of 4-bromo-phenethylamine
[0071]
[0072] Add 1000g of p-bromoacetophenone and 950g of ammonium formate into a 5L reaction flask, and slowly heat to 120°C. The two raw materials slowly dissolve and become liquid. Start stirring and keep at 120°C for 2 hours. Then the reaction temperature was raised to 180° C. and kept at this temperature for 5 hours. Stop the reaction, after cooling, add water, dichloromethane, separate layers, concentrate the organic phase, add 670 ml of concentrated hydrochloric acid and 1000 ml of water, reflux, 1-2 hours, the reactant first has solids, then clarifies, and then a large amount of solids are precipitated , the reaction is over, after cooling slightly, add 1000 milliliters of toluene, and cool to room temperature under stirring. Filtration, the solid was fully washed twice with toluene, and the solid was dried to obtain the hydrochloride salt of the product, which was neutralized with sodium hydroxide, extracted wi...
Embodiment 3
[0084] (1) Preparation of 4-bromo-phenethylamine
[0085]
[0086] Add 1,000g of p-bromoacetophenone and 1,900g of ammonium formate into a 5L reaction flask, and slowly heat to 120°C. The two raw materials slowly dissolve and become liquid, start stirring, and keep at 120°C for 2 hours. Then the reaction temperature was raised to 180° C. and kept at this temperature for 5 hours. Stop the reaction, after cooling, add water, dichloromethane, separate layers, concentrate the organic phase, add 670 ml of concentrated hydrochloric acid and 1000 ml of water, reflux, 1-2 hours, the reactant first has solids, then clarifies, and then a large amount of solids are precipitated , the reaction is over, after cooling slightly, add 1000 milliliters of toluene, and cool to room temperature under stirring. Filtration, the solid was fully washed twice with toluene, and the solid was dried to obtain the hydrochloride salt of the product, which was neutralized with sodium hydroxide, extracte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com