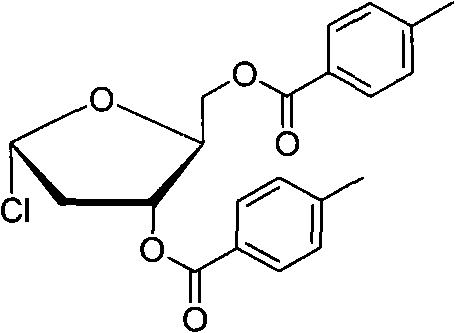
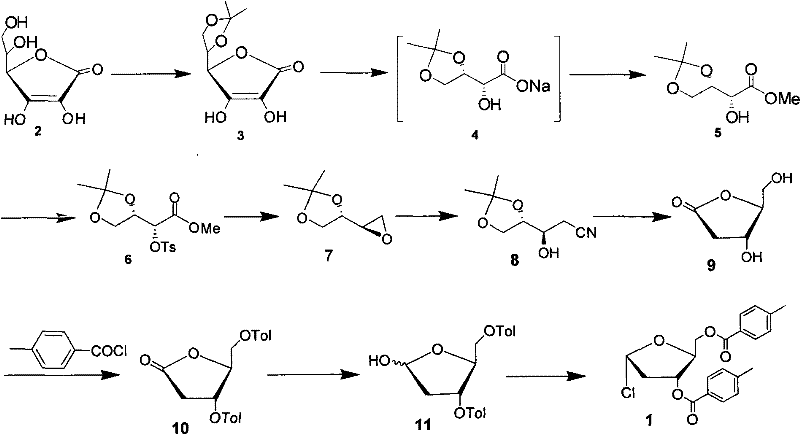
Technology for preparing key intermediate of telbivudine
A technology for telbivudine and intermediates, which is applied in the preparation of sugar derivatives, sugar derivatives, esterified saccharides, etc., can solve the problems of expensive raw materials and high cost, and achieves easy availability of raw materials, low cost and high yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
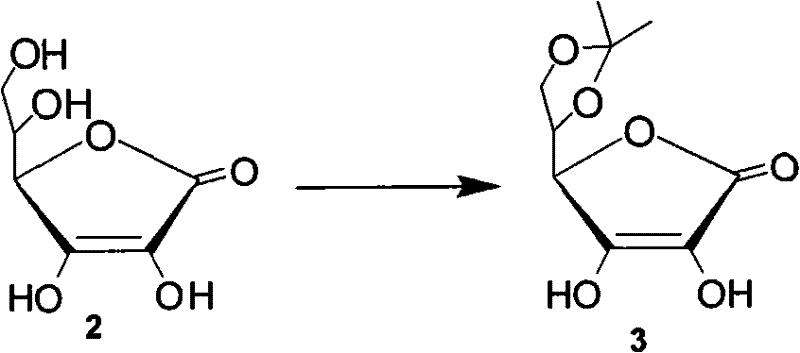
[0026] Dissolve 200g (1.12mol) of vitamin C (compound 2), 88ml (1.2mol) of acetyl chloride in 500ml of acetone, heat up to 40°C, keep stirring for 2 hours, cool the reaction solution to 0°C, filter, and dissolve in 100ml of acetone Wash the filter cake, and then dry the filter cake to obtain 230 g of compound (3) (yield: 95%): white solid; mp: 212-214°C [lit: 218-219°C]; 1 HNMR (250MHz, D 2 O), δ1.38 (s, 6H), 4.18 (dd, J=9.1, 5.0, 2.1H), 4.32 (dd, J=9.1, 7.2Hz, 1H), 4.60 (ddd, J=7.2, 5.0, 2.2Hz, 1H), 4.93(d, J=2.2Hz, 1H)
Embodiment 2
[0028] Add compound (3) (220g, 1mol) and 500ml deionized water into a 2L reaction flask, add 30% NaOH solution (75ml) dropwise under stirring, and add NaHCO 3 (215g, 2.56mol), then slowly add 30% hydrogen peroxide (200ml, 2.1mol) dropwise, and stir at room temperature for 1 hour after dropping.
[0029] Sodium sulfite (12.6g, 0.1mol) and sodium bicarbonate (105g, 1.25mol) were added to the reaction solution, and the temperature was raised to 40°C, and dimethyl sulfate (375ml, 4mol) was added dropwise. At room temperature, extract with dichloromethane (500ml×2), combine the organic layers, dry over anhydrous magnesium sulfate, filter, and concentrate to obtain 173g of compound (5) (yield: 90%): colorless oil; 1 H NMR (250MHz, CDCl 3 )δ1.28(s, 3H), 1.36(s, 3H).3.07(d, J=8.1Hz, 1H), 3.83(s, 3H), 3.99-4.17(m, 3H), 4.40(td, Jt =6.7Hz, Jd=2.8Hz, 1H)
Embodiment 3
[0031] Weigh 130g (0.68mol) of compound (5) and 15ml of triethylamine into a 500ml reaction bottle, add 100ml of dichloromethane and stir to dissolve, cool down to 0-5°C in an ice-water bath, add 129g (0.68mom) of p-toluenesulfonyl chloride dropwise 200ml of dichloromethane solution, after dropping, raised to room temperature and stirred for 6 hours, washed with water until neutral, dried the organic layer, and concentrated under reduced pressure to obtain 220g of compound (6), with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com