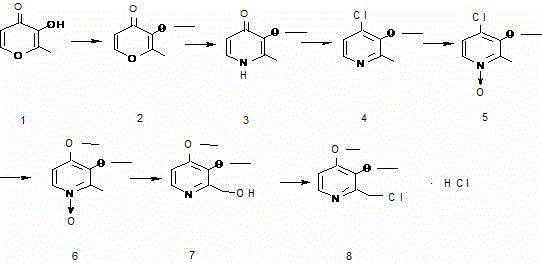
Preparation method of pantoprazole intermediate 2-chloromethyl-3,4-dimethoxy pyridine hydrochloride
A technology of dimethoxypyridine hydrochloride and dimethoxypyridine, which is applied in the field of preparation of pantoprazole intermediate 2-chloromethyl-3,4-dimethoxypyridine hydrochloride, can Solve the problems of low safety, complex reaction, and many by-products, and achieve the effect of improving work efficiency, reducing reaction steps, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 3-hydroxy-2-methyl-4-pyridone.
[0021] Add 40g of 3-hydroxy-2-methyl-4-pyrone and 200g of ammonia water (20%) into the reaction bottle, slowly heat up to dissolve, keep at 40°C for 2h, slowly add 30g of ammonium carbonate, keep at 40°C for 2h, slowly add 62g of ammonium carbonate, kept at 40°C for 3h, slowly raised to reflux and maintained at reflux for 5h, then cooled to 0°C, filtered with suction and rinsed with a small amount of water, dried to obtain 38g of 3-hydroxy-2-methyl-4-pyridone ( Yield 95.7%); MS: m / z = 125.2 [M] + . 1H NMR ([D6]DMSO, 400 Hz): δ 2.17 (s, 3H, CH 3 ), 6.07–6.10(d,1H,5-H), 7.38–7.57 (d, 1H, 6-H), 11.4 (s, 1H, NH).
Embodiment 2
[0022] Example 2: Preparation of 3-hydroxy-2-methyl-4-pyridone.
[0023] Add 40g of 3-hydroxy-2-methyl-4-pyrone and 120g of methanol to the reaction flask, slowly heat up to dissolve, slowly add 120g of ammonium bicarbonate at 40°C, keep warm at 40°C for 2h, slowly add 90g of ammonium bicarbonate, Insulate for 3 hours, slowly raise the temperature to reflux and maintain reflux for 5 hours, then cool down to -10°C, filter with suction and rinse with a small amount of ice methanol, and dry to obtain 25g of 3-hydroxy-2-methyl-4-pyridone (yield 63.1%) ; MS: m / z = 125.2 [M] + . 1H NMR ([D6]DMSO, 400 Hz): δ 2.17 (s, 3H, CH 3 ), 6.07–6.10(d,1H,5-H), 7.38–7.57 (d, 1H, 6-H), 11.4 (s, 1H, NH).
Embodiment 3
[0024] Example 3: Preparation of 3-hydroxy-2-methyl-4-pyridone.
[0025] Add 40g of 3-hydroxy-2-methyl-4-pyrone and 100ml of water to the reaction flask, heat up to dissolve, slowly add 153g of ammonium bicarbonate at 40°C, keep warm at 40°C for 3h, slowly add 90g of ammonium bicarbonate, Insulate for 5 hours, slowly raise the temperature to reflux and maintain reflux for 5 hours, then cool down to 0°C, filter with suction and rinse with a small amount of water, and dry to obtain 30g of 3-hydroxy-2-methyl-4-pyridone (yield 75.6%);
[0026] MS: m / z = 125.2 [M] + . 1H NMR ([D6]DMSO, 400 Hz): δ 2.17 (s, 3H, CH 3 ), 6.07–6.10(d,1H,5-H), 7.38–7.57 (d, 1H, 6-H), 11.4 (s, 1H, NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com