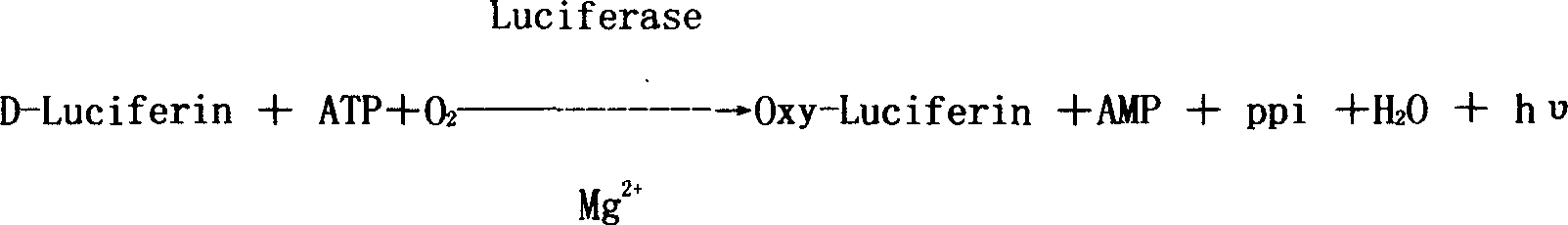Chemical synthetic process of D-fluorescein and device therefor
A technology of chemical synthesis and fluorescein, which is applied in organic chemistry and other fields, can solve the problems of harsh reaction conditions, difficulty in control, and low total yield of D-fluorescein, and achieve the effect of high luminescence coefficient and efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: About the simple and convenient synthesis device of D-fluorescein
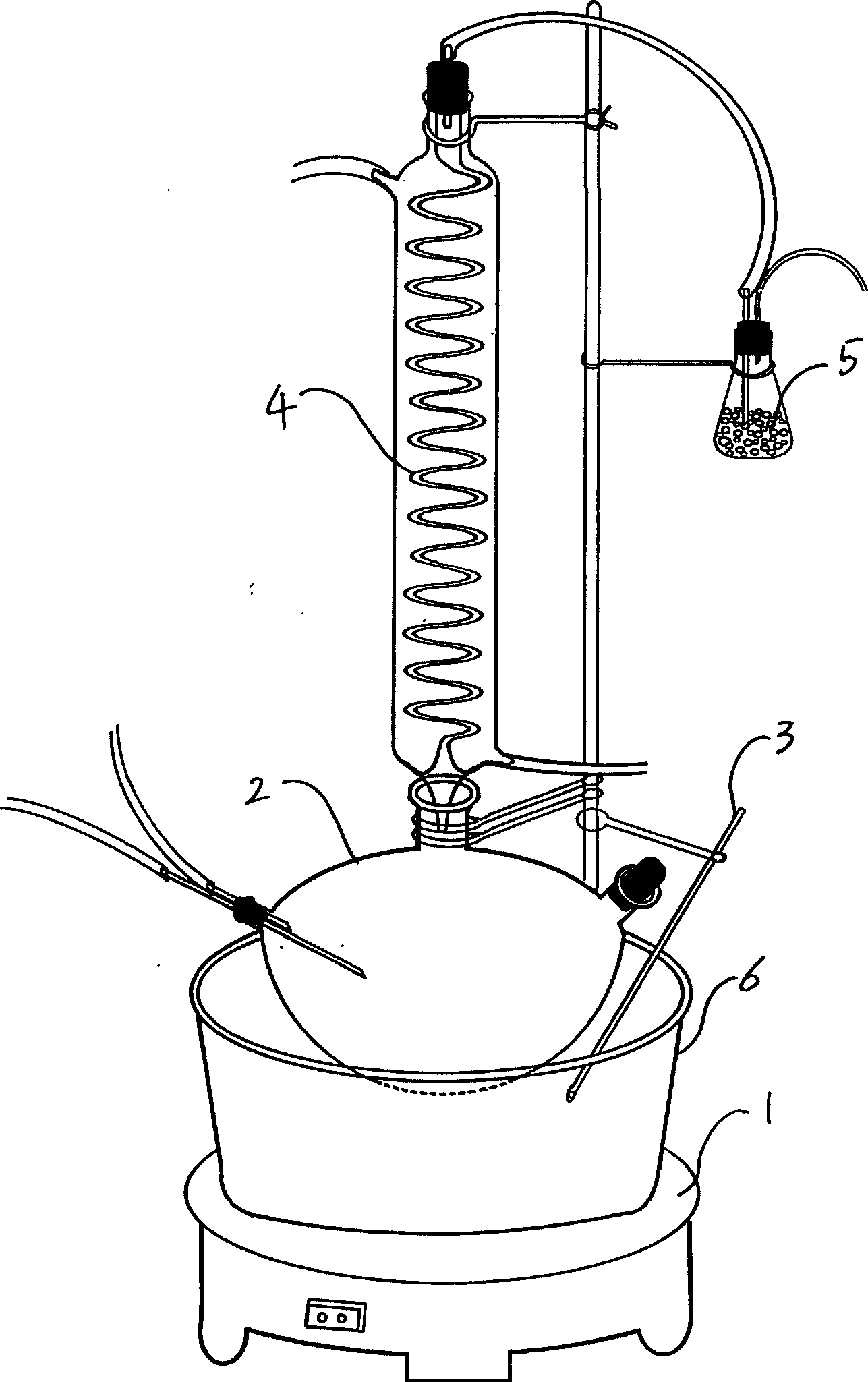
[0030] The reaction device consists of a temperature control device that can be adjusted more accurately in a wide range (0-300°C), a high-temperature oil bath heating system, a vacuum pumping nitrogen gas regulating system, and three ports with a condenser tube 4 and a waste gas absorption device. Flask 2 reaction system composition. The entire reaction device was placed in a fume hood except for the nitrogen supply bottle, vacuum device and temperature control device.
[0031] like figure 1 As shown, the high-temperature oil bath system can be used to hold castor oil (about 2000mL) in a stainless steel pot 6 on an adjustable electric furnace 1 (500W), and finely adjust the temperature through a temperature sensor 3; The opening is used for vacuuming and nitrogen gas, the middle opening is used for sample addition and cooling cycle, and the right opening is used for sampling detection (...
Embodiment 2
[0032] Embodiment 2: about the synthetic method of D-fluorescein
[0033] 1. Demethylation reaction
[0034] Weigh 940mg 2-cyano-6-methoxybenzothiazoe (2-Cyano-6-methoxybenzothiazoe) (99%) and 1000~3060mg pyridine hydrochloride (Pyridine hydrochloride) (98%) and put them into a small test tube, draw Put nitrogen into a temperature-adjustable high-temperature oil-bath reaction bottle after vacuuming, and keep warm at 190-220°C for 1-3 hours, stop heating to terminate the reaction, and extract the target product of demethylation after cooling.
[0035] 2. Extraction of demethylation target product
[0036] Add 200mL-400mL ice water to the above-mentioned reaction tube, and add 100mL-200mL ethyl acetate. The organic layer was washed once with ~400 mL of ice water, combined and dried by a rotary vacuum evaporator to obtain 1480 mg of a yellow-red substance.
[0037] 3. Purification of demethylated products
[0038]Add 80-120 mesh coarse-pore silica gel to an organic pressuriza...
Embodiment 3
[0052] Embodiment 3: about the synthetic method of D-fluorescein
[0053] 1. Methyl reaction (2002, 6 / 17)
[0054] Weigh 949mg of 2-cyano-6-methoxybenzothiazoe (2-Cyano-6-methoxybenzothiazoe) (99%) and 1000mg of pyridine hydrochloride (Pyridine hydrochloride) (98%) into a small test tube, vacuum After nitrogen gas, put it into a temperature-adjustable high-temperature oil-bath reaction flask, keep it at 200°C for 1 hour, stop heating to terminate the reaction, and extract the target product of demethylation after cooling.
[0055] 2. Extraction of methyl target product
[0056] Add 200mL of ice water to the above reaction tube, and add 100mL of ethyl acetate, shake well, extract the organic layer with a separatory funnel, extract the water layer twice with 100mL of ethyl acetate, wash the organic layer once with 200mL of ice water , after combining the organic layers, dried by a rotary vacuum evaporator to obtain 776 mg of a yellow-red substance.
[0057] 3. Purification of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com