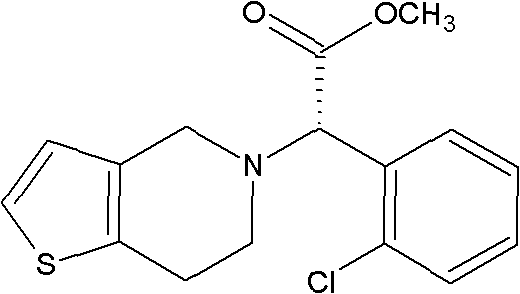
Method for preparing clopidogrel
A clopidogrel and ortho-chlorine technology, applied in the chemical field, can solve the problem of high cost and achieve the effects of low production cost, good environmental protection and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
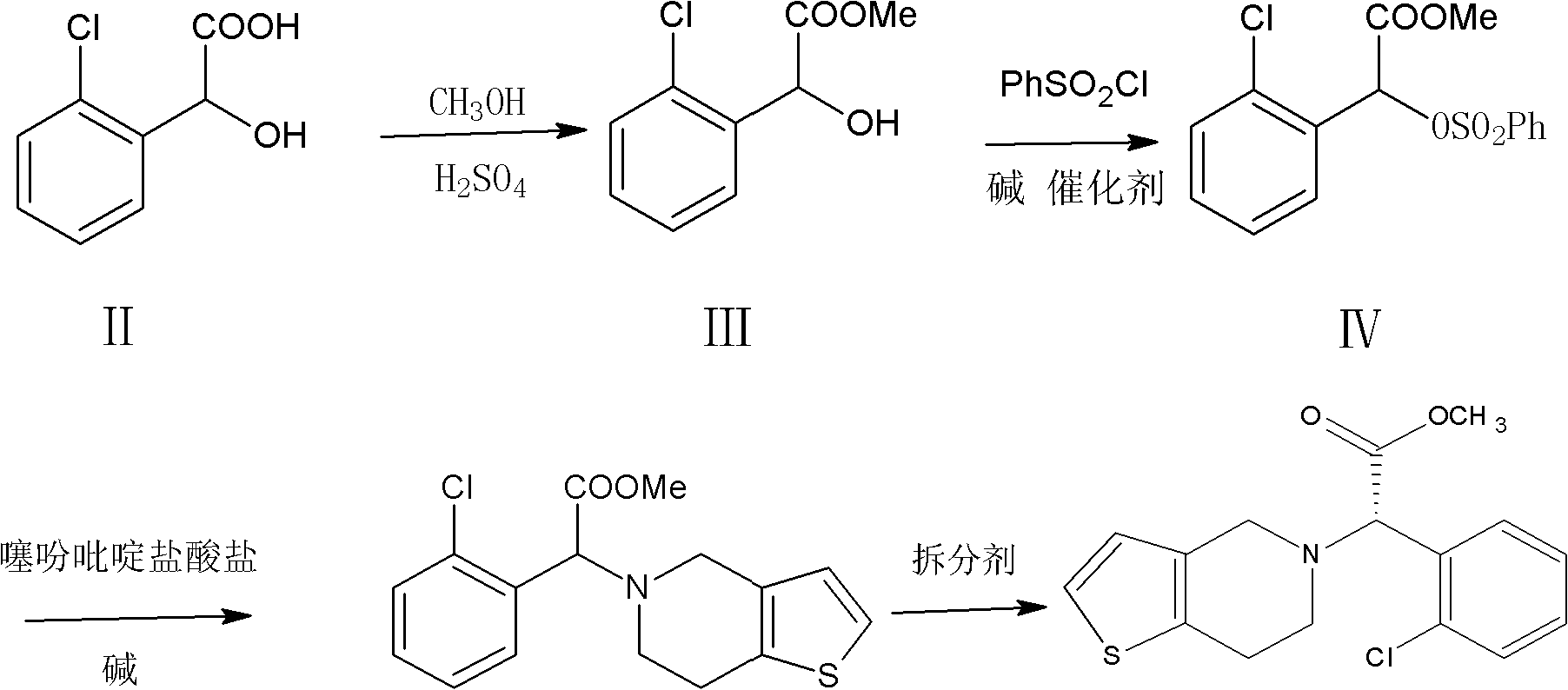
[0025] a) Synthesis of R, S-methyl o-chloromandelic acid (Ⅲ)
[0026] Put 20.5g of R,S-o-chloromandelic acid (0.11mol), 130ml of methanol and 4g of concentrated sulfuric acid into the reaction flask, raise the temperature to 60-65°C, and keep the reaction temperature for 3h. After the reaction is completed, the methanol is recovered to dryness, 100 ml of dichloromethane and 40 ml of potassium carbonate aqueous solution (15%) are added to the oily residue, washed with water, allowed to stand, and the organic layer is separated for use in the next reaction.
[0027] b) Synthesis of the compound 2-benzenesulfonate-2 (2-chlorophenyl) methyl acetate (Ⅳ)
[0028] Add 30g tri-n-propylamine (0.21mol) to the dichloromethane solution of R,S-methyl o-chloromandelic acid prepared in step a), stir, cool to 0~5℃ with chilled brine, slowly add 25g benzene dropwise Sulfonyl chloride (0.14mol), incubate the reaction for about 3h until the reaction is complete. After that, add 10ml of 30% hydrochlor...
Embodiment 2
[0034] a) Synthesis of R, S-methyl o-chloromandelic acid (Ⅲ)
[0035] This step is the same as in Example 1
[0036] b) Synthesis of the compound 2-tosylate-2(2-chlorophenyl) methyl acetate (Ⅳ)
[0037] Add 25 g of triethylamine (0.25 mol) to the R, S-methyl o-chloromandelic acid obtained from the above reaction, stir, pass chilled salt water to cool to 0~5℃, and start to add p-35 g toluenesulfonyl chloride solution (0.18 mol), the reaction is incubated for 3h, and the end of the reaction is controlled by TLC. After that, add the hydrochloric acid aqueous solution, stir and wash, stand still, separate into layers, and wash the oil layer to pH 6-7. After completion, the dichloromethane was recovered to obtain 36 g of oil (0.10 mol), the cumulative molar yield of the two-step reaction was 92%, and the liquid content of the product was 97.9%.
[0038] c) Synthesis of R, S-clopidogrel (Ⅴ)
[0039] Add 100ml of butyl acetate, 94g of potassium carbonate aqueous solution (30%), and 30g of t...
Embodiment 3
[0043] a) Synthesis of R, S-methyl o-chloromandelic acid (Ⅲ)
[0044] This step is the same as in Example 1
[0045] b) Synthesis of the compound 2-tosylate-2(2-chlorophenyl) methyl acetate (Ⅳ)
[0046] This step is the same as in Example 2
[0047] c) Synthesis of R, S-clopidogrel (Ⅴ)
[0048] Add 100 ml of chloroform, 94 g of potassium carbonate aqueous solution (30%), and 30 g of thiophenepyridine hydrochloride (0.17 mol) to the formula IV obtained from the above reaction, stir at 20-30°C for 1 hour, and then heat up to 50°C for 6 hours. After finishing, let stand, separate into layers, wash the oil layer with water, separate the oil layer, distill and recover the chloroform to dryness, obtain 29.3g R, S-clopidogrel (0.091mol), the molar yield is 90.1%
[0049] d) Synthesis of S-clopidogrel (Ⅰ)
[0050] To 29.3g of R,S-clopidogrel free base (0.091mol) prepared in step c) was added 100ml of acetone and 50ml of methyl tert-butyl ether and 23g of L-camphorsulfonic acid (0.099mol). The t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com