Preparation method of benzbromarone
A technology of benzbromarone and ethylbenzene, applied in the field of chemical synthesis, can solve the problems of strong bromine corrosion, incomplete reaction, low reaction temperature, etc., and achieves improved reaction conditions, improved purification methods, and strong corrosiveness. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
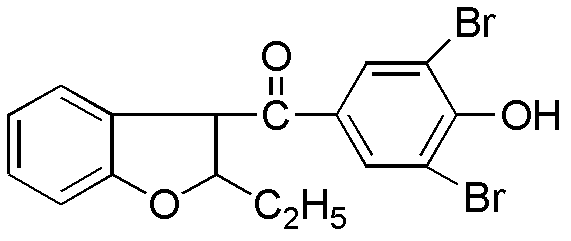
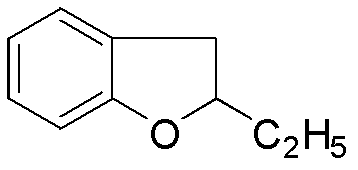
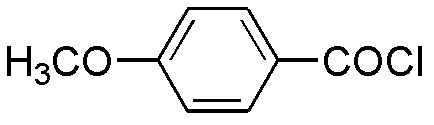
[0020] In a 1000mL reaction flask equipped with mechanical stirring, add 60g of 2-ethylbenzofuran, 75g of p-methoxybenzoyl chloride, and 500mL of dichloromethane. Add tin 110g, drop it in about 40 minutes, the color of the reaction solution turns red, and a brown-red solid gradually appears, keep the temperature at 0±3°C and continue stirring for 3 hours. After the reaction is over, slowly add 200 mL of ice water and 125 mL of aqueous hydrochloric acid (52.5 mL of concentrated hydrochloric acid) under stirring at 0°C to adjust the pH of the reaction solution to 1-2, then add 200 mL of dichloromethane for extraction, wash with water, Alkali washing, salt washing, anhydrous magnesium sulfate drying. After suction filtration and concentration, 110 g of yellow oil was obtained, with a yield of 95.8%.
Embodiment 2
[0016] Figure 5 It is the structural formula diagram of 2-ethyl-3-p-hydroxybenzoyl-benzofuran of the present invention.
Embodiment 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com