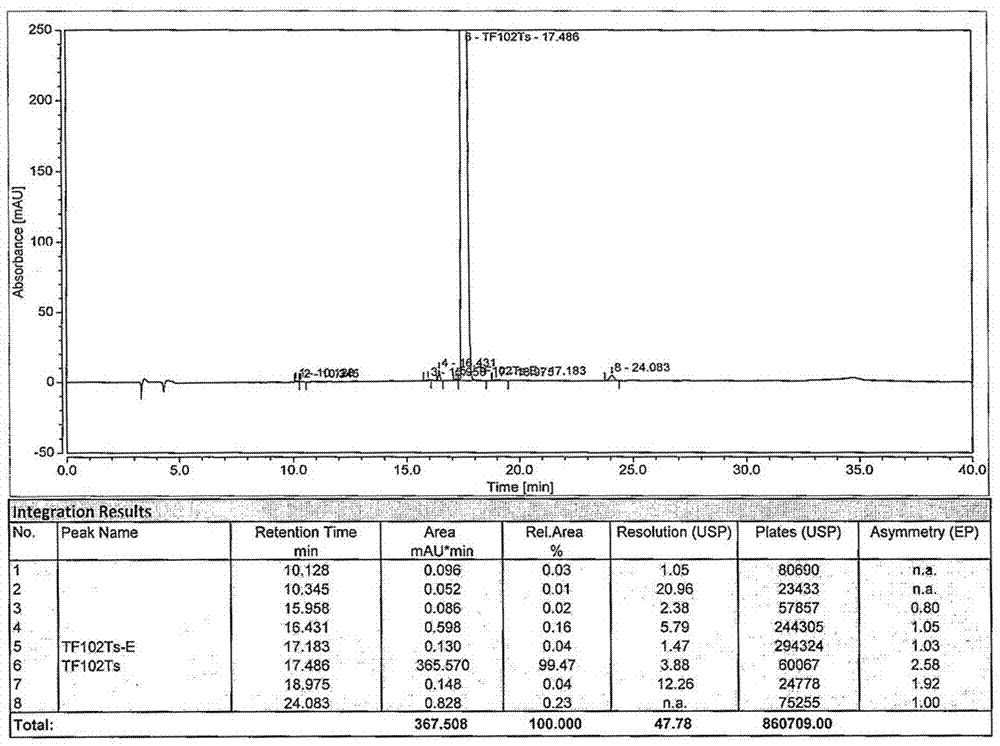
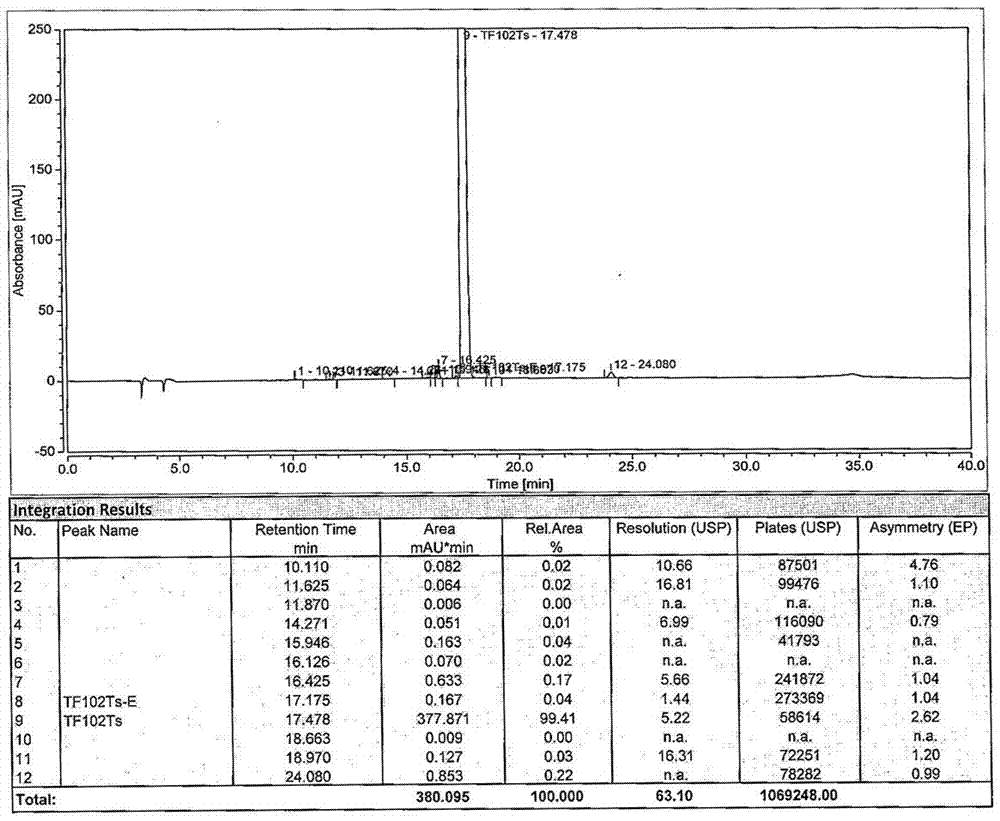
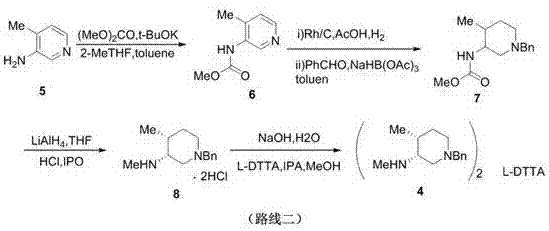
Preparation method of tofacitinib citrate starting material
A technology of tofacitinib and raw materials, applied in the field of chemical pharmacy, can solve the problems of large-scale industrial waste water, increase environmental protection pressure, high price, etc., and achieve the effects of reducing production costs, reducing safety risks, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Synthesis of Compound IV
[0036]Add 50g (1eq) of 4-methylpyridine, 200mL of acetone and 102g (1.5eq) of benzyl chloride into a 500mL reaction flask, start stirring, and keep the reaction for 4 hours; TLC monitors that the reaction is basically complete, cool down to room temperature, and store at an external temperature of 40~45°C Concentrate under reduced pressure to dryness, then add 200mL of acetonitrile to the concentrated solution, raise the temperature to reflux until the solution is clear, then slowly cool down to 0~5°C, stir at this temperature for 2h, filter with suction, and filter the cake with cooled acetonitrile Washed twice, dried to obtain a white solid, and the filter cake was vacuum-dried at 40-45°C to obtain 106.1 g of a white powder, with a yield of 90%.
[0037] 2. Synthesis of Compound V
[0038] Add 100g of compound VI (1.0eq) and 400mL of ethanol to a 1L reaction flask, start stirring, and after cooling down to 15°C, add NaBH to the reaction ...
Embodiment 2
[0048] 1. Synthesis of Compound IV
[0049] Add 100g (1eq) of 4-methylpyridine, 400mL of acetone and 204g (1.5eq) of benzyl chloride into a 1L reaction flask, start stirring, and keep the reaction for 4h; TLC monitors that the reaction is basically complete, cool down to room temperature, and store at an external temperature of 40~45°C Concentrate under reduced pressure to dryness, then add 400mL of acetonitrile to the concentrated solution, raise the temperature to reflux until the solution is clear, then slowly cool down to 0~5°C, stir at this temperature for 2h, filter with suction, and filter the cake with a cooled Washed twice with acetonitrile, drained to obtain a white solid, the filter cake was vacuum-dried at 40-45°C to obtain 212.1 g of white powder, yield 90%.
[0050] 2. Synthesis of Compound V
[0051] Add 200g of compound VI (1.0eq) and 800mL of ethanol to a 2L reaction flask, start stirring, and after cooling down to 15°C, add NaBH to the reaction solution 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com