Preparation method of esomeprazole magnesium
A technology of esomeprazole magnesium and compounds, which is applied in the field of medicinal chemistry, can solve the problems of unfavorable large-scale industrial production, low resolution yield, high production cost, etc., and achieve easy industrial production, good reproducibility, and stable form Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a kind of preparation method of esomeprazole magnesium, comprising:
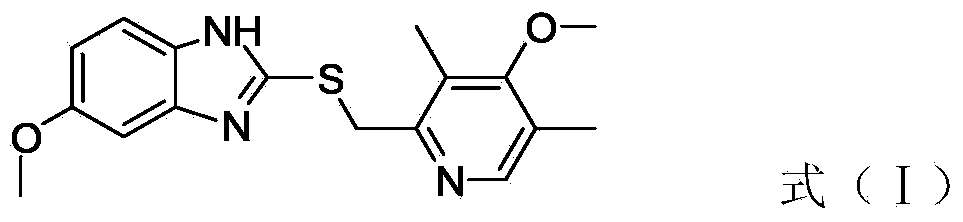
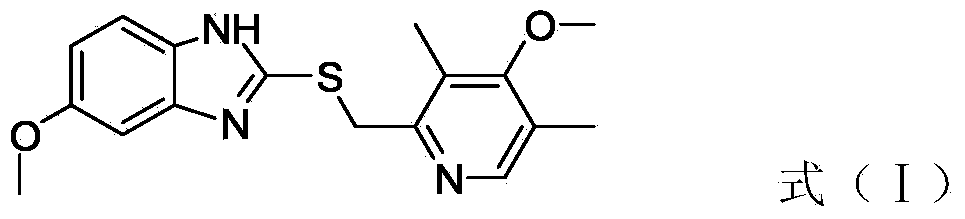
[0026] A, 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride, inorganic base and 2-mercapto-5 methoxy-benzimidazole react in ethanol to obtain the compound with (I ) compounds with structure
[0027]
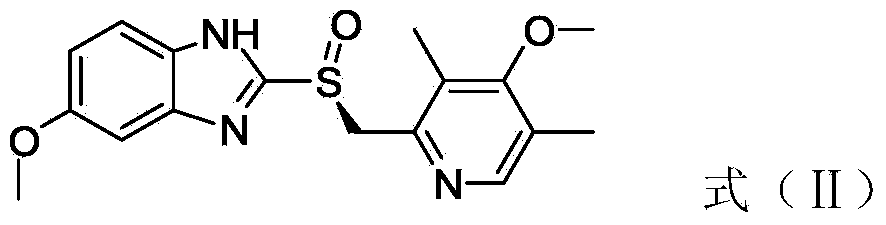
[0028] b. React the compound with the structure of formula (I) with catalyst, chiral ligand (+)-l-tartrate-di-tert-butyl ester, organic base, water and oxidant in an organic solvent to obtain the compound with structure (II) ,
[0029]
[0030] c. reacting the compound having the structure of formula (II) with the inorganic sodium salt in an organic solvent to obtain esomeprazole sodium salt.
[0031] In the present invention, 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and 2-mercapto-5methoxy-benzimidazole are first dissolved in ethanol, and then an inorganic base is added to reflux After 3-6 hours, the compound having the structure of formula (I...
Embodiment 1-1
[0039] Preparation of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]-lH-benzimidazole
[0040] 2-Chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (50g, 225mmol), sodium carbonate (53g, 495mmol) and 2-mercapto-5-methoxy Base-benzimidazole (40g, 225 mmol) was added in a three-necked flask (1000 ml), and absolute ethanol (400 ml) was measured by a graduated cylinder, heated to reflux, stirred for 4 hours, and monitored by TLC at the end of the reaction (developing agent was acetic acid Ethyl ester) after the reaction is complete, stop the heating, cool down to room temperature naturally, add diatomaceous earth (15g), remove the insoluble matter by suction filtration under reduced pressure, collect the filtrate, and spin evaporate under reduced pressure to obtain an oily substance, which is recrystallized with toluene to obtain a white powdery solid (67.6g).
[0041] The purity of the compound with the structure of formula (I) measured by HPLC is 99.8%
Embodiment 1-2
[0043] Preparation of esomeprazole
[0044] Add the compound of formula (I) obtained above (67.6g, 205mmol) into a three-necked flask, measure toluene (500ml) into the three-necked flask, heat up to 80°C, heat and stir to dissolve, then cool down to 50°C, Add (+)-l-tartrate-di-tert-butyl ester (21.5g, 82mmol), tetraisopropyl titanate (11.6g, 41mmol) was added, and pure water (0.36g, 20.5mmol) was added dropwise ), keep the reaction temperature at about 50°C, stir for 1 hour, then cool down to 30-35°C, add N,N-diisopropylethylamine (15.9g, 123mmol) dropwise, after the drop, add isopropyl Hydrogen peroxide (44.6g, 205mmol) was added dropwise to the reaction system, and the reaction temperature was maintained at 30-35°C during the dropwise addition. Aqueous sodium hydroxide solution (250ml) was quenched, separated and purified to give the compound of formula (II) (29.7g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com