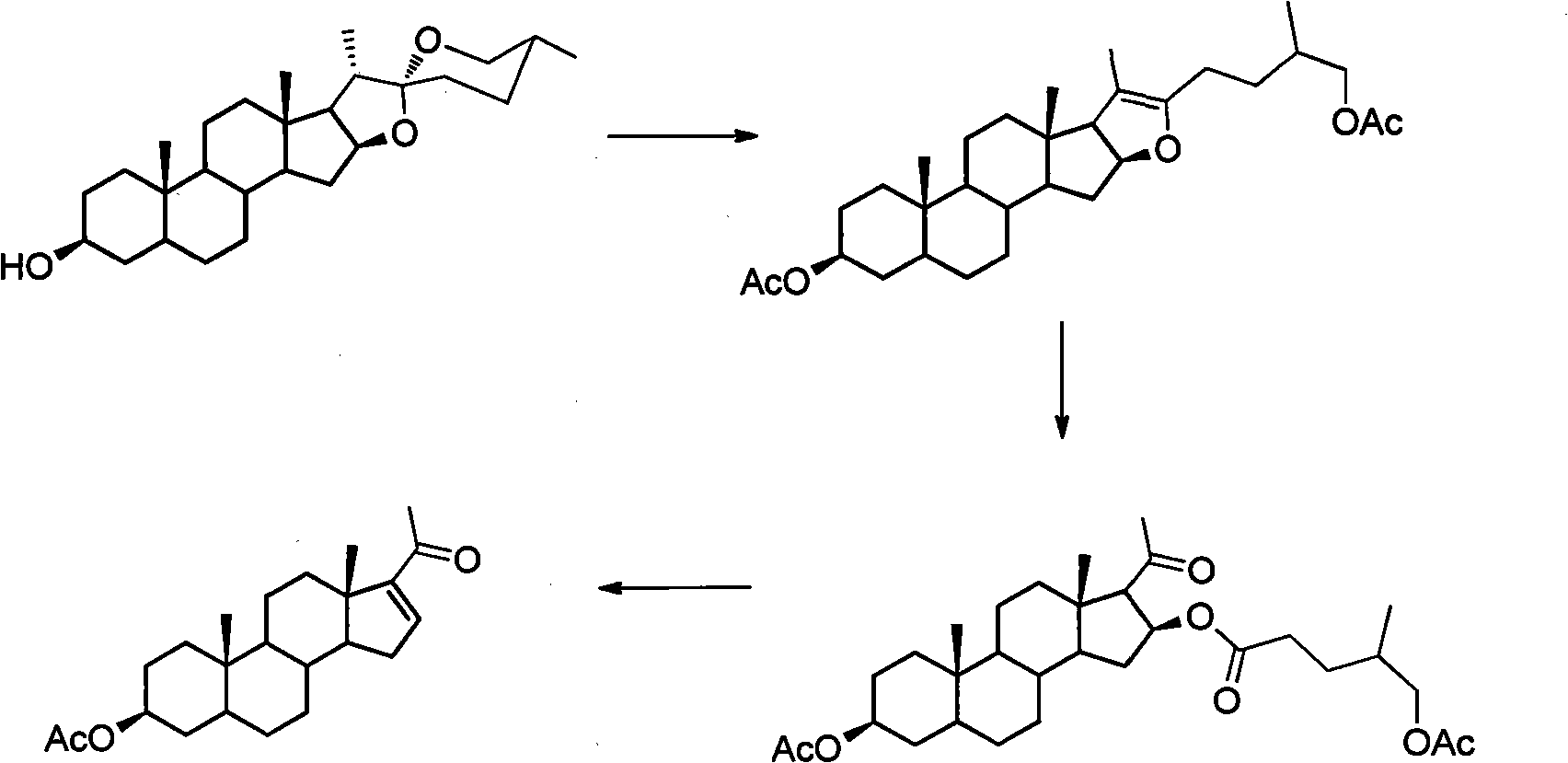
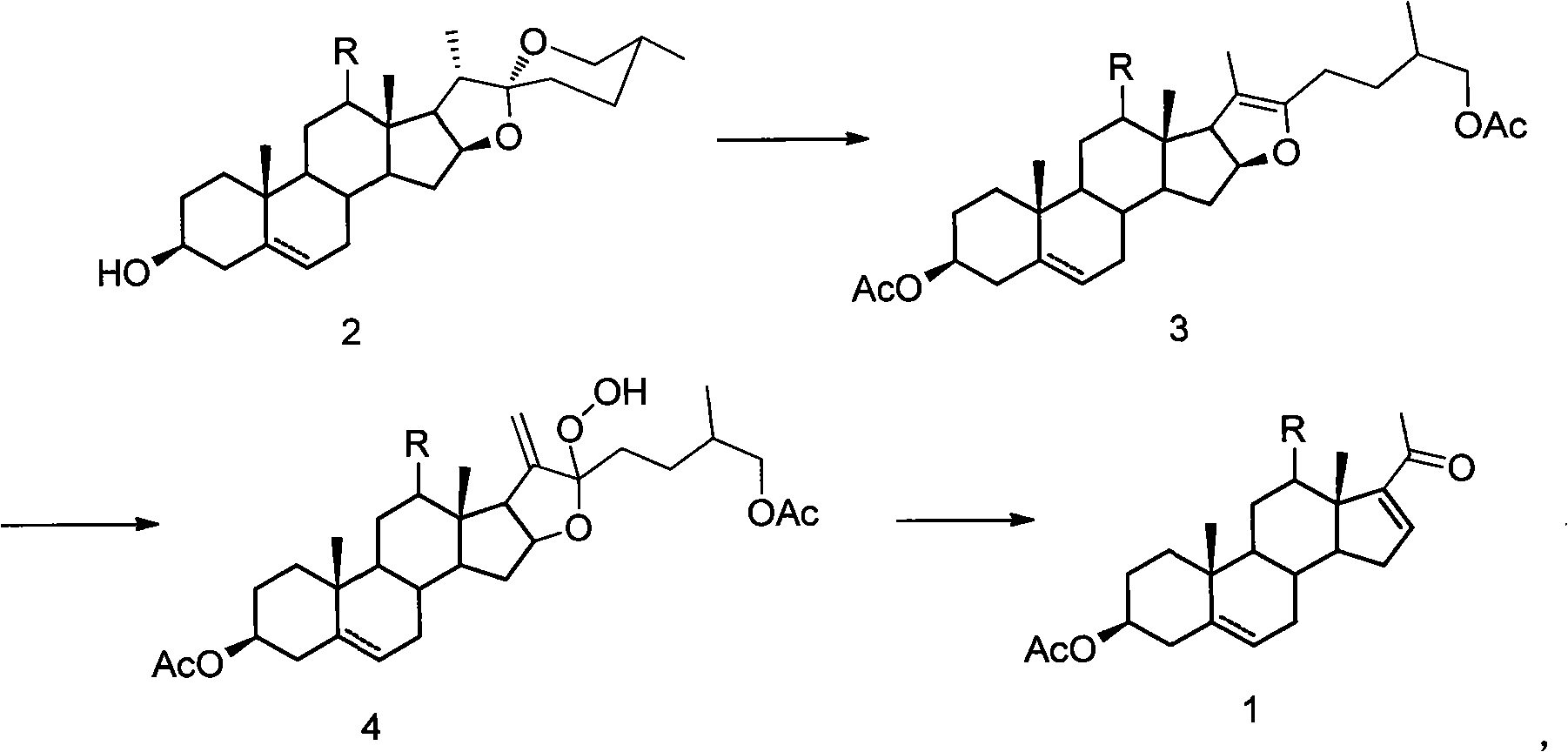
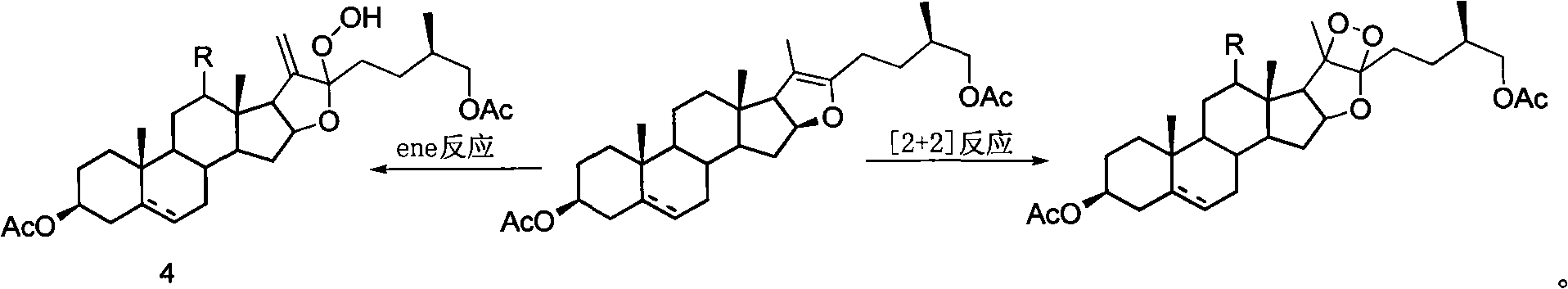
Method for synthesizing pregnenolol acetate and congener thereof
A kind of technology of pregnenone alcohol acetate and synthetic method, which is applied in the direction of steroidal compounds and organic chemistry, can solve the problems of unfavorable mass production of pregnenone alcohol acetate, environmental pollution, and low three-step total yield, and achieve mild conditions , Simple equipment and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis of Example 1 Compound 1a
[0019]
[0020] 100 g of compound 2a, 150 ml of acetic anhydride and 52 ml of acetic acid were reacted at a pressure of 0.6 MPa and a temperature of 200° C. for 1 hour. Transfer the reaction solution to a distillation flask, recover the unreacted acetic anhydride and acetic acid under reduced pressure, dissolve the residue with 21 cyclohexane, then add 0.7 g of tetraphenylporphyrin, react with incandescent lamp for 3 hours and complete the reaction of all raw materials , add 1.31ml of concentrated sulfuric acid, heat to reflux and stir for 14 hours and then the reaction is complete. After cooling, wash with water and saturated aqueous sodium carbonate solution, then with saturated saline, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and recrystallize from ethanol to obtain compound 1a 64.5 g, the yield is 75%.
[0021] 1 H-NMR (400MHz, CDCl 3 ( d, 2H, J=8Hz), 1.06(s, 3H), 0.92(s, 3H).
Embodiment 2
[0022] Synthesis of Example 2 Compound 1a
[0023]
[0024] 100 g of compound 2a, 150 ml of acetic anhydride and 52 ml of acetic acid were reacted at a pressure of 0.6 MPa and a temperature of 200° C. for 1 hour. Transfer the reaction solution to a distillation flask, recover the unreacted acetic anhydride and acetic acid under reduced pressure, dissolve the residue with 21 g of ethyl acetate, then add 1.2 g of rose bengal, and react for 2.5 hours under incandescent lamp irradiation until all the raw materials are completely reacted. After recovery of ethyl acetate under reduced pressure, 1 l of acetic acid was added, heated to reflux and stirred for 13 hours, and cyclohexane was added for extraction. The cyclohexane extract was washed with saturated sodium bicarbonate water and brine, the solvent was evaporated under reduced pressure, and recrystallized with ethanol to obtain 63 g of compound 1a with a yield of 72%.
Embodiment 3
[0025] Synthesis of Example 3 Compound 4a
[0026]
[0027] 100 g of compound 2a, 150 ml of acetic anhydride and 52 ml of acetic acid were reacted at 0.6 MPa and 200° C. for 1 hour. Transfer the reaction solution to a distillation flask, recover the unreacted acetic anhydride and acetic acid under reduced pressure, dissolve the residue with 21 cyclohexane, then add 0.7 g of tetraphenylporphyrin, react with incandescent lamp for 3 hours and complete the reaction of all raw materials , cyclohexane was distilled off under reduced pressure, and 10.2 g of compound 4a was isolated with a yield of 80%.
[0028] 1 H-NMR (400MHz, CDCl 3 ( m, 1H), 3.95-3.88(m, 2H), 2.74-2.72(d, 1H, J=8Hz), 2.03-2.04(d, 6H), 1.03(s, 3H), 0.94-0.92(d, 3H , J=8Hz), 0.74(s, 3H).
[0029] ESI-Ms: 553.3 [M+Na + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com