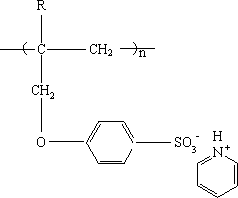
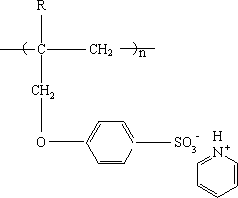
Cation exchange membrane and preparation method thereof
A technology of cation exchange membrane and membrane liquid, applied in the field of cation exchange membrane and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of intermediates:
[0027] Add 15.12g (0.28mol) sodium methoxide to a dry four-necked flask, and add 88ml methanol to dissolve it completely; put 54.88g (0.28mol) sodium p-hydroxybenzenesulfonate dissolved in 400ml methanol into the dropping funnel 1 solution, the dropping funnel 2 was filled with 38.72g (0.32mol) 3-bromopropene; at room temperature, the liquid in the two dropping funnels was slowly added dropwise to the methanol solution of sodium methoxide. ℃, reflux reaction for 4 hours; after the solution is cooled, filter to remove insoluble matter and impurities, distill methanol under reduced pressure, add a small amount of water to the flask to dissolve the solid, cool to 0°C, recrystallize and dry to obtain a white solid powder.
[0028] (2) Monomer synthesis:
[0029] Weigh 15.81g (0.067mol) of the above white solid powder and 7.4g of N-methylpyrrolidone into a dry four-neck flask, stir, add 8.7g (0.075mol) of pyridine hydrochloride, heat up to ...
Embodiment 2
[0038] (1) Synthesis of intermediates:
[0039] Add 15.12g (0.28mol) sodium methoxide to a dry four-necked flask, and add 88ml methanol to dissolve it completely; put 54.88g (0.28mol) sodium p-hydroxybenzenesulfonate dissolved in 400ml methanol into the dropping funnel 1 solution, the dropping funnel 2 was filled with 38.72g (0.32mol) 3-bromopropene; at room temperature, the liquid in the two dropping funnels was slowly added dropwise to the methanol solution of sodium methoxide. ℃, reflux reaction for 4 hours; after the solution is cooled, filter to remove insoluble matter and impurities, distill methanol under reduced pressure, add a small amount of water to the flask to dissolve the solid, cool to 0°C, recrystallize and dry to obtain a white solid powder.
[0040] (2) Monomer synthesis:
[0041] Weigh 14.16g (0.06mol) of the above white solid powder and 11g of N-methylpyrrolidone into a dry four-neck flask, stir, add 7.8g (0.068mol) of pyridine hydrochloride, raise the tem...
Embodiment 3
[0050] (1) Synthesis of intermediates:
[0051] Add 15.12g (0.28mol) sodium methoxide to a dry four-necked flask, and add 88ml methanol to dissolve it completely; put 54.88g (0.28mol) sodium p-hydroxybenzenesulfonate dissolved in 400ml methanol into the dropping funnel 1 solution, the dropping funnel 2 was filled with 33.88g (0.28mol) 3-bromopropene; at room temperature, the liquid in the two dropping funnels was slowly added dropwise to the methanol solution of sodium methoxide. ℃, reflux for 3 hours; after the solution is cooled, remove insoluble matter and impurities by filtration, distill methanol under reduced pressure, add a small amount of water to the flask to dissolve the solid, cool to 0°C, recrystallize and dry to obtain a white solid powder.
[0052] (2) Monomer synthesis:
[0053] Weigh 15.81g (0.067mol) of the above white solid powder and 7.4g of N-methylpyrrolidone into a dry four-neck flask, stir, add 7.77g (0.067mol) of pyridine hydrochloride, heat up to 85°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Membrane resistance | aaaaa | aaaaa |
| Membrane resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com