Preparation method of rabeprazole sodium crystal type compound
The technology of rabeprazole sodium and compound is applied in the field of preparation of rabeprazole sodium crystal compound, can solve the problems of high price, limited consumer groups, etc., and achieves the effects of low cost, low manufacturing cost and stable property of goods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
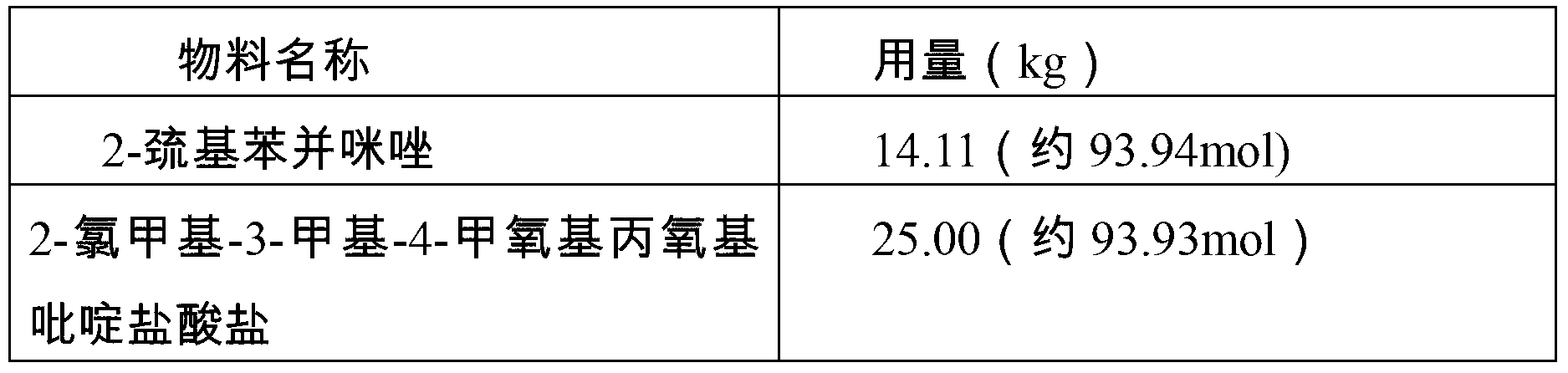
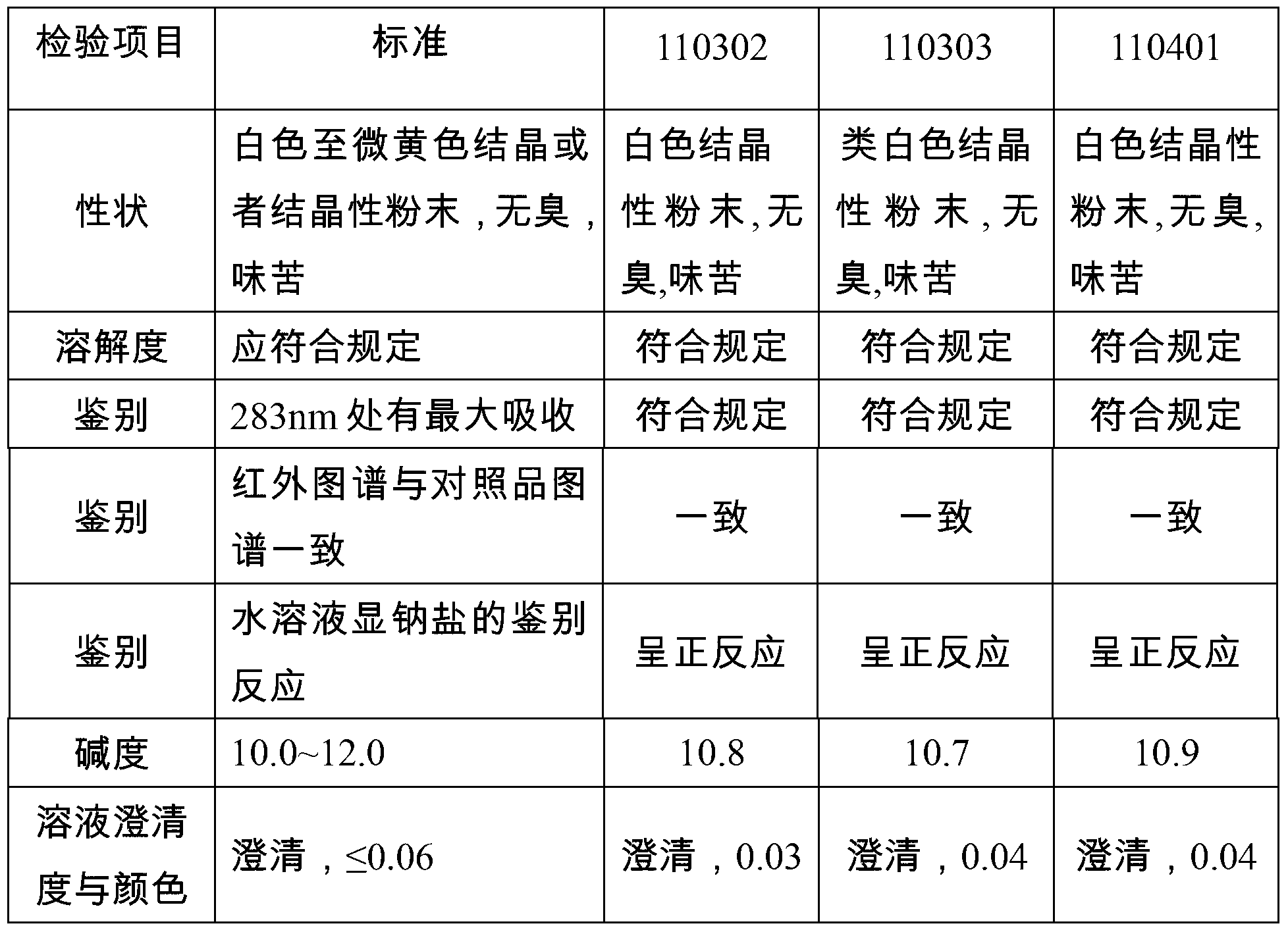
[0027] This example provides a preparation method of rabeprazole sodium crystal form compound, which is 2-mercaptobenzimidazole, 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride As a raw material, the final product is obtained through condensation, oxidation and salt-forming reactions, which includes the following preparation steps:
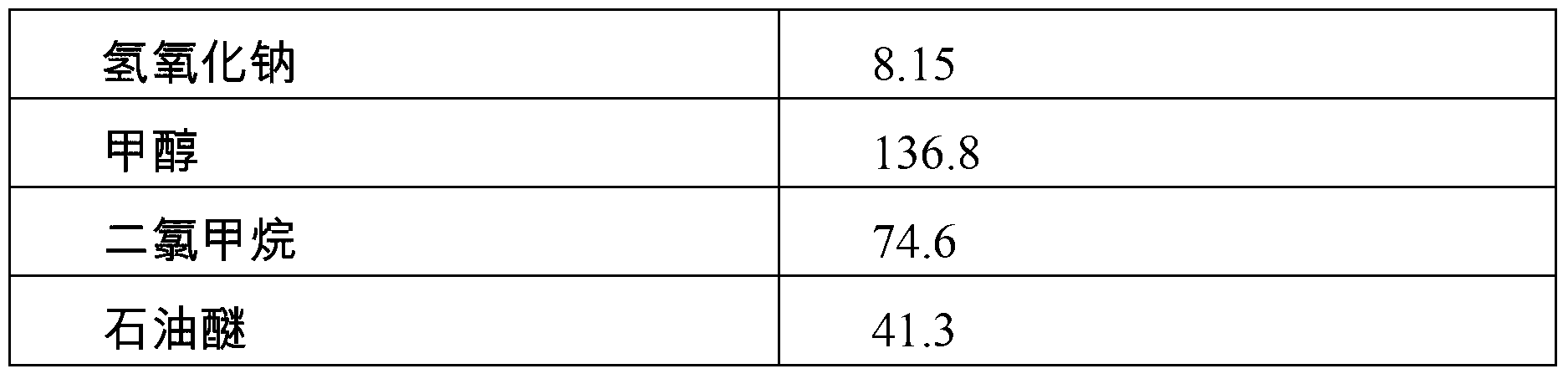
[0028] Condensation reaction: Prepare 32.6kg of methanol in the first clean reaction tank, stir at a speed of 35Hz, drop in 25.00kg of 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride, stir Dissolve and prepare a methanol solution of 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride for later use.
[0029] Prepare 98.8kg of methanol in the second clean reaction tank, add 8.15kg of sodium hydroxide under stirring at a speed of 40Hz, stir evenly and control the temperature at 20-25°C, put in 14.11kg of 2-mercaptobenzimidazole, and heat up to 50-60°C , add the methanol solution of 2-chloromethyl-3-methyl-4-m...
Embodiment 2
[0040] This example provides a preparation method of rabeprazole sodium crystal form compound, which is 2-mercaptobenzimidazole, 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride As a raw material, the final product is obtained through condensation, oxidation and salt-forming reactions, which includes the following preparation steps:
[0041] Condensation reaction: Prepare 32.6kg of methanol in the first clean reaction tank, stir at a speed of 35Hz, drop in 22.8kg of 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride, stir Dissolve and prepare a methanol solution of 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride for later use.
[0042] Prepare 81.5kg of methanol in the second clean reaction tank, add 6.52kg of sodium hydroxide under stirring at a speed of 40Hz, stir evenly and control the temperature at 20-25°C, put in 8.15kg of 2-mercaptobenzimidazole, and heat up to 50-60°C , add the methanol solution of 2-chloromethyl-3-methyl-4-met...
Embodiment 3
[0046] This example provides a preparation method of rabeprazole sodium crystal form compound, which is 2-mercaptobenzimidazole, 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride As a raw material, the final product is obtained through condensation, oxidation and salt-forming reactions, which includes the following preparation steps:
[0047] Condensation reaction: Prepare 32.6kg and 29.3kg of A in the first clean reaction tank, stir and dissolve, and prepare a methanol solution of 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride, spare.
[0048] Prepare 98.8kg of methanol in the second clean reaction tank, add 9.88kg of sodium hydroxide under stirring at a speed of 40Hz, stir evenly and control the temperature at 20-25°C, put in 19.76kg of 2-mercaptobenzimidazole, and heat up to 50-60°C , add the methanol solution of 2-chloromethyl-3-methyl-4-methoxypropoxypyridine hydrochloride prepared above at a temperature of 50-60°C, and add in about 30 minute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com