Preparation method of esomeprazole and preparation method of esomeprazole salt
A technology of esomeprazole salt and lutidine hydrochloride, applied in the field of medicine, can solve the problems of high price of the resolving agent, difficult to realize industrialized production, low selectivity and the like, and achieves reduction of impurity nitrogen oxides and The production of sulfone, easy industrial production, the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The invention provides a kind of preparation method of esomeprazole salt, comprising:
[0037] a, 2-chloromethyl-4-nitro-3,5-lutidine hydrochloride, inorganic base and 5-methoxy-2-mercaptobenzimidazole are reacted in water to obtain formula (I) structural compounds,
[0038] Formula (I);
[0039] b. Reacting the compound having the structure of formula (I), a chiral inducer, a catalyst, water and an oxidizing agent in an organic solvent to obtain a compound having the structure of formula (II),
[0040] Formula (II);
[0041] c. reacting the compound having the structure of formula (II) and methoxide in an organic solvent to obtain esomeprazole salt, and the methoxide is sodium methoxide or potassium methoxide.
[0042] The present invention reacts 2-chloromethyl-4-nitro-3,5-lutidine hydrochloride, inorganic base and 5-methoxy-2-mercaptobenzimidazole in water to obtain ) structure, the molar ratio of the 2-chloromethyl-4-nitro-3,5-lutidine hydrochloride to the 5...
Embodiment 1
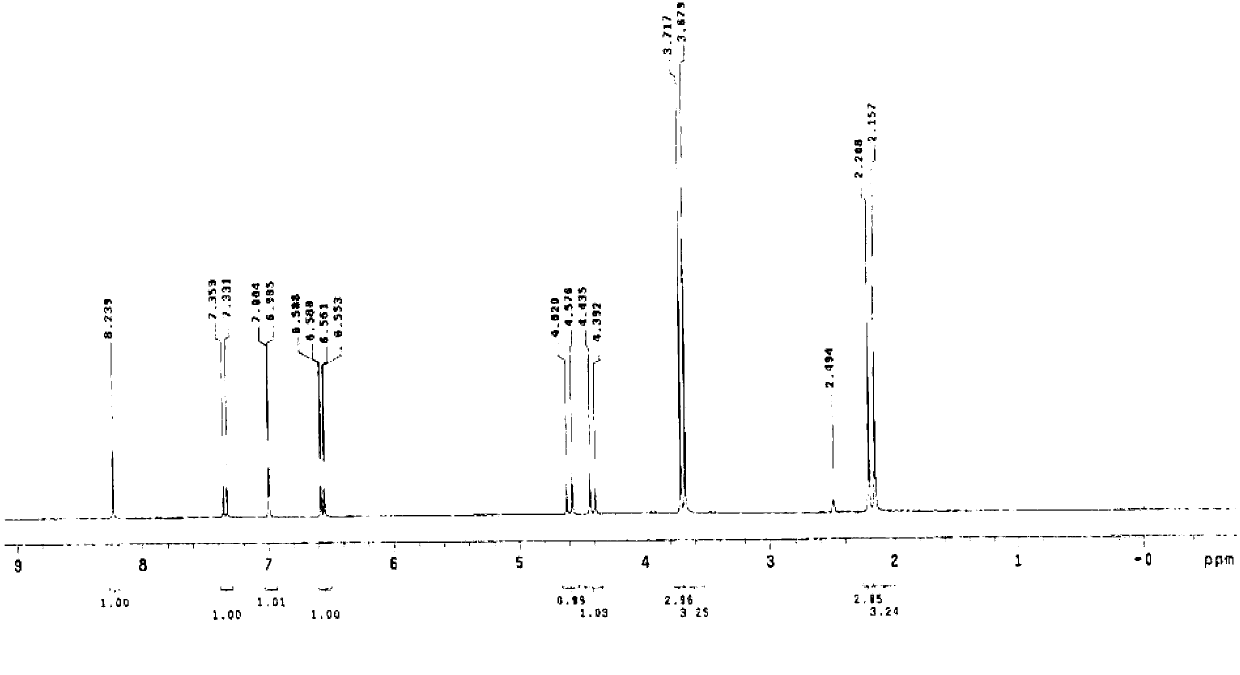
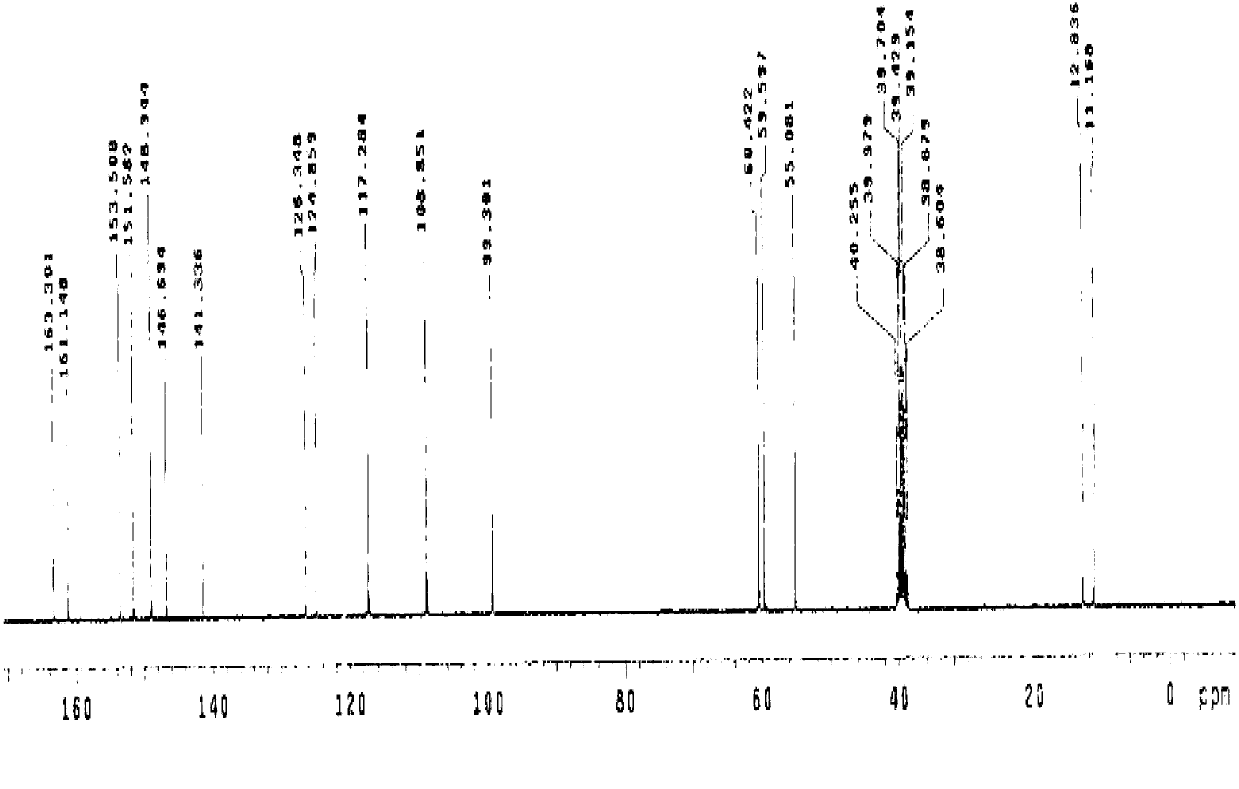
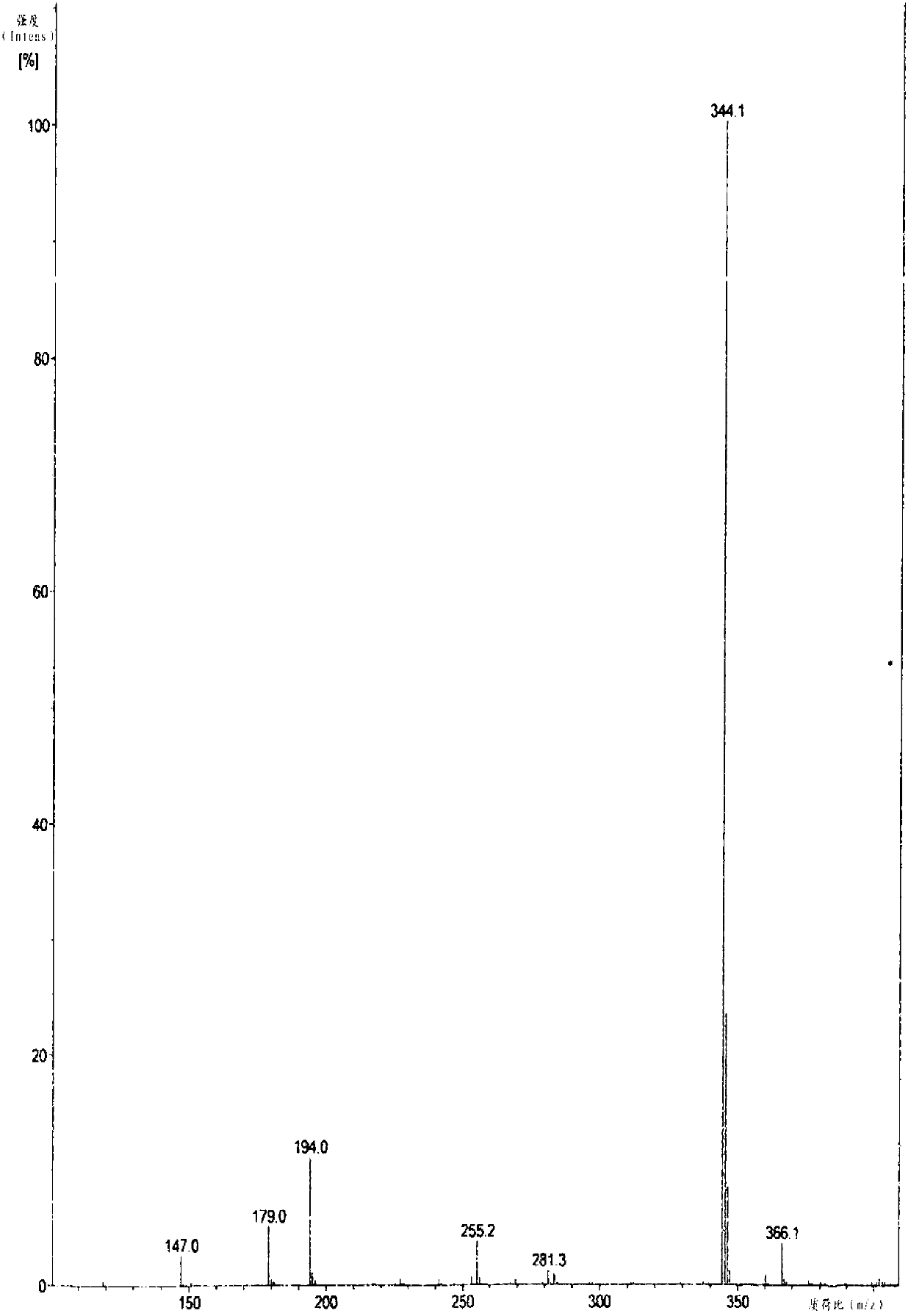
[0069] (S)-5-methoxy-2-[[4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole sodium (Esso Meprazole sodium) preparation
[0070] Add 23.7 g (100 mmol) of 2-chloromethyl-4-nitro-3,5-lutidine hydrochloride into 95 ml of water, heat up to 40°C, stir to dissolve and set aside; Take 100 ml of tap water and pour it into a 500 ml three-neck bottle, add 8.8 g (220 mmol) of caustic soda, and then add 19.8 g (110 mmol) of 5-methoxy-2-mercaptobenzimidazole into the above dilute alkaline water and stir Make it dissolve, then control the temperature at 35~40°C, slowly drop 2-chloromethyl-4-nitro-3,5-lutidine hydrochloride solution into the reaction solution over 3 hours, and add Afterwards, continue to react at 35~40°C for 3 hours, and determine the end point of the reaction by TLC (developing agent is V 甲苯 :V 乙酸乙酯 :V 甲醇 =5:2:1), after the reaction was completed, filter, wash and dry to obtain 34.1 grams of the compound with the structure of formula (I).
[0071] The pu...
Embodiment 2
[0081] (S)-5-methoxy-2-[[4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole potassium (Esso Meprazole potassium) preparation
[0082] Add 23.7 g (100 mmol) of 2-chloromethyl-4-nitro-3,5-lutidine hydrochloride into 95 ml of water, heat up to 40°C, stir to dissolve and set aside; Take 100 ml of tap water and pour it into a 500 ml three-neck bottle, add 8.8 g (220 mmol) of caustic soda, and then add 19.8 g (110 mmol) of 5-methoxy-2-mercaptobenzimidazole into the above dilute alkaline water and stir Make it dissolve, then control the temperature at 35~40°C, slowly drop 2-chloromethyl-4-nitro-3,5-lutidine hydrochloride aqueous solution into the reaction solution over 3 hours, add After completion, continue to react at 35~40°C for 3 hours, and determine the end point of the reaction by TLC (developing agent is V 甲苯 :V 乙酸乙酯 :V 甲醇 =5:2:1), after the reaction was completed, filter, wash and dry to obtain 34.2 grams of the compound with the structure of formula (I). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com