Antitumor agent for acute myeloid leukemia
A technology of myeloid leukemia and anti-tumor agent, which is applied in the field of anti-tumor agent for acute myeloid leukemia, and can solve the problems that no research has been carried out.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
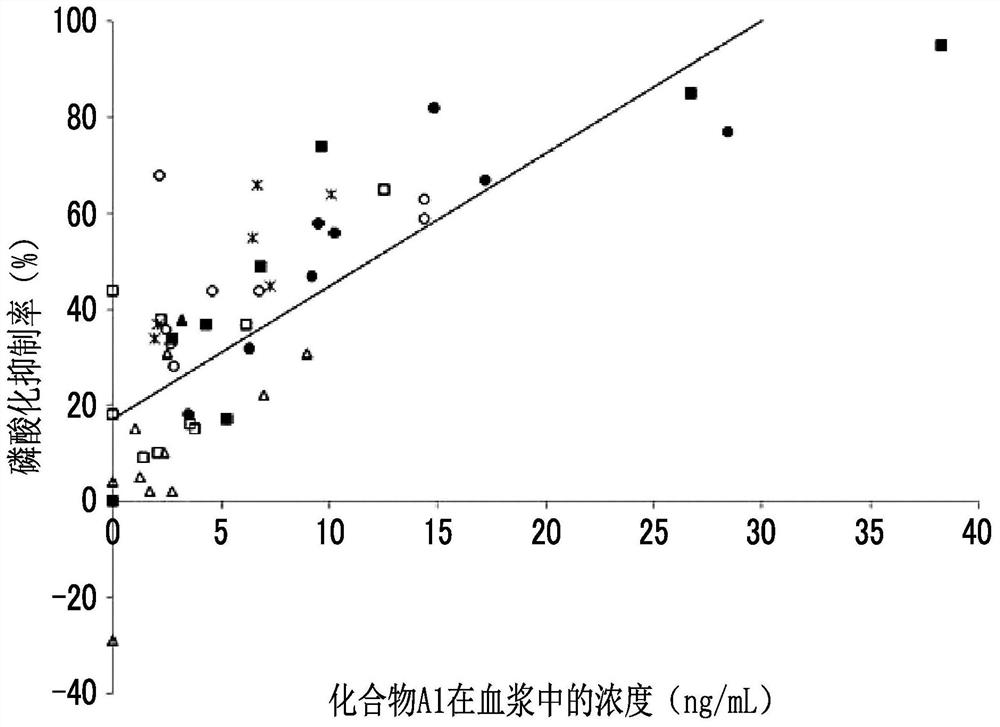
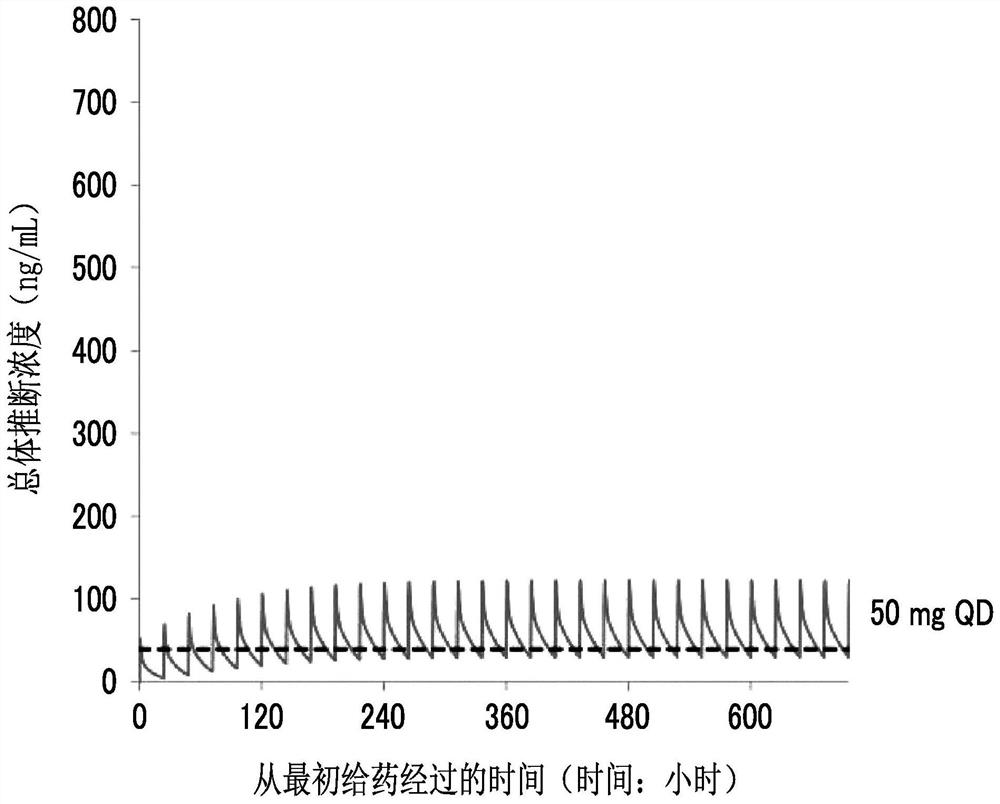
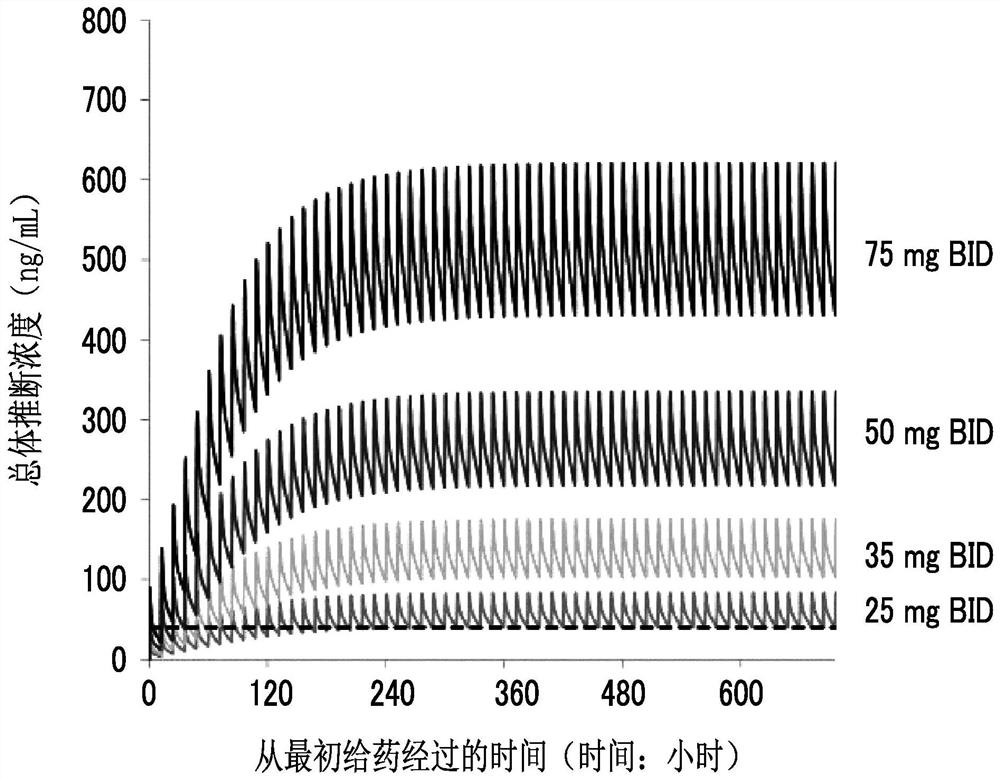
[0285] Population pharmacokinetic analysis was performed using plasma concentration data of 198 compounds A1 obtained from 10 acute myelogenous leukemia patients. As the population pharmacokinetic analysis software, NONMEM (registered trademark) (ICON Development Solutions Co., Ltd., software version 7.3) was used. A QD of 50 mg once a day (QD) will be implemented in order to study the dose that can achieve a plasma drug concentration exceeding 40 ng / mL (the target value of the expected drug effect) using the parameters estimated from the population pharmacokinetic analysis During the administration, the simulation results of the simulation of drug concentration changes when 25 to 75 mg BID was administered twice a day and 10 to 75 mg TID were administered three times a day are shown in Figure 2 ~ Figure 4 . exist Figure 2 ~ Figure 4 In , the dashed line represents the line at 40 ng / mL.
[0286] As a result of the simulation, it was predicted that the dose of the overall ...
Embodiment 2
[0288]
[0289] For patients with acute myelogenous leukemia, compound A1 was administered twice a day, 25-225 mg once, BID before meals. Good effects (for example, a reduction in the ratio of blast cells in the bone marrow, a therapeutic effect of PR or higher) were confirmed. The specific dosage is 50mg, 75mg, 100mg or 150mg each time.
[0290] As the initial treatment, patients received chemotherapy based on cytarabine, daunorubicin, idarubicin, etc., and some patients did not achieve CR, CRi, CRp, or PR after the initial treatment.
[0291]
[0292] The therapeutic effect was judged by the following criteria.
[0293] The bone marrow aspiration subjects were evaluated and judged by the following criteria.
[0294]CR (Complete Response): 5% or less of bone marrow blast cells with no Auer rods confirmed, and the number of neutrophils and platelets is 1,000 / μL or more and 100,000 / μL or more state.
[0295] CRp (Complete Response with incomplete platelet recovery): Bon...
Embodiment 3
[0300]
[0301] For patients with acute myelogenous leukemia, compound A1 was administered TID in an amount of 20-150 mg three times a day before meals. Good effects (for example, reduction of blast cell ratio in bone marrow, therapeutic effect of PR or higher) were confirmed.
[0302] As the initial treatment, patients received chemotherapy based on cytarabine, daunorubicin, idarubicin, etc., and some patients failed to achieve CR, CRi or CRp after the initial treatment.
[0303]
[0304] For patients with acute myelogenous leukemia, compound A1 was administered QD before meals in an amount of 50-300 mg once a day. More specifically, the dosage is 50, 75, 100, 150, 225, 300 mg. A relatively good effect (for example, a reduction in the blast cell ratio in the bone marrow, a therapeutic effect of PR or higher) was confirmed.
[0305] As the initial treatment, patients received chemotherapy based on cytarabine, daunorubicin, idarubicin, etc., and some patients failed to ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com