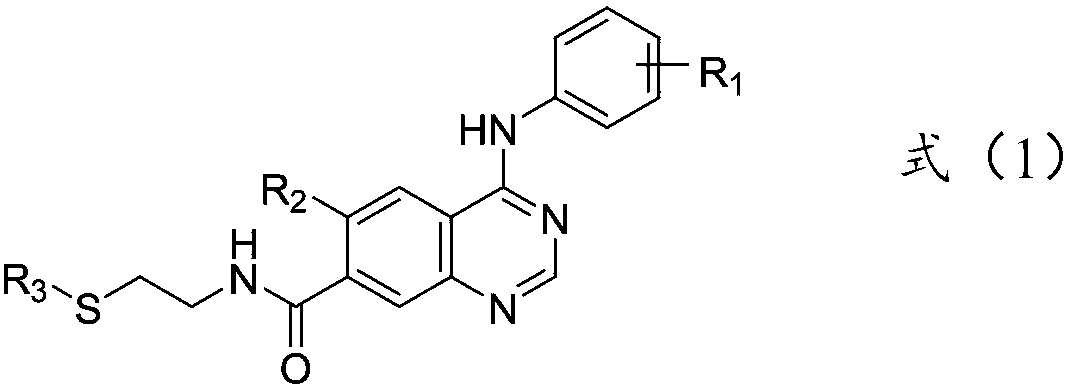
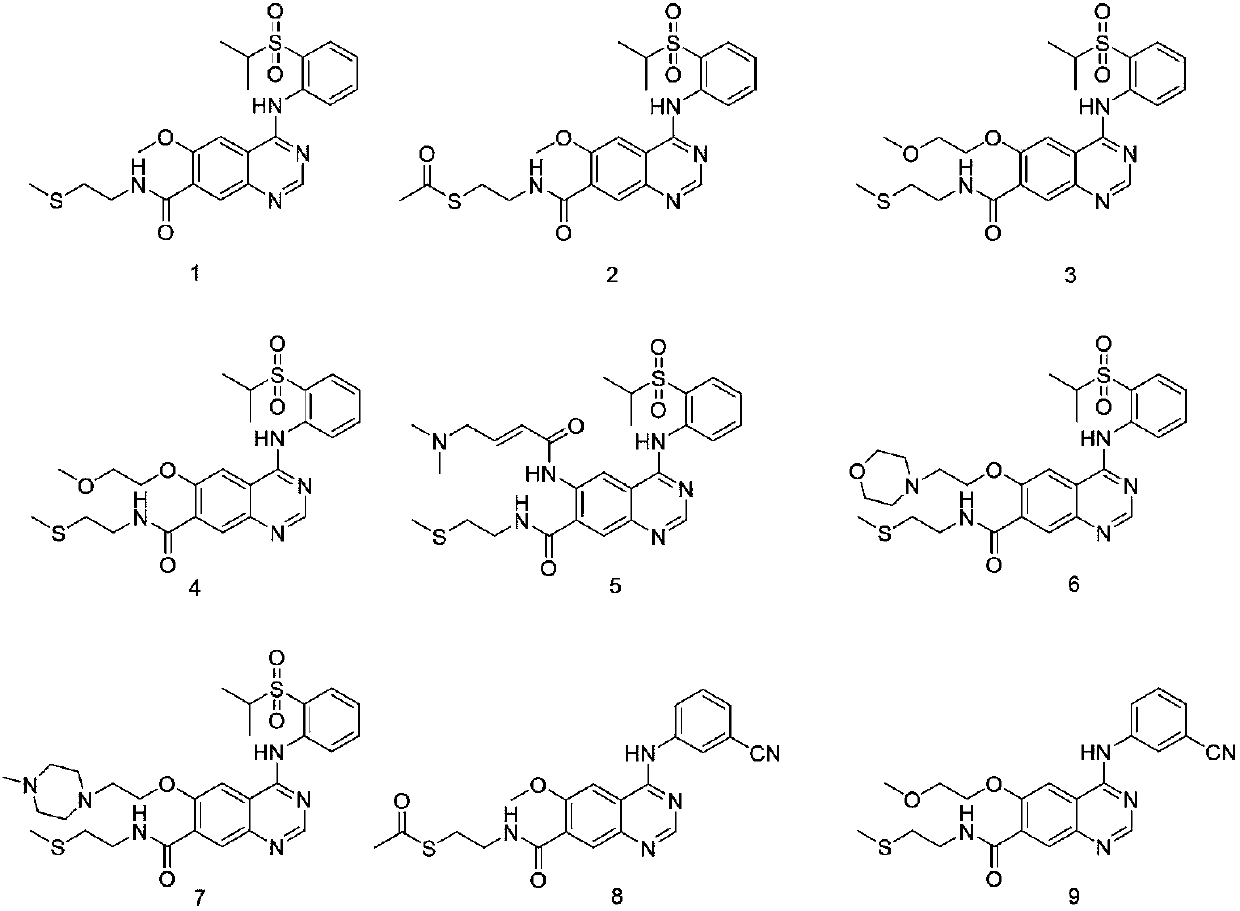
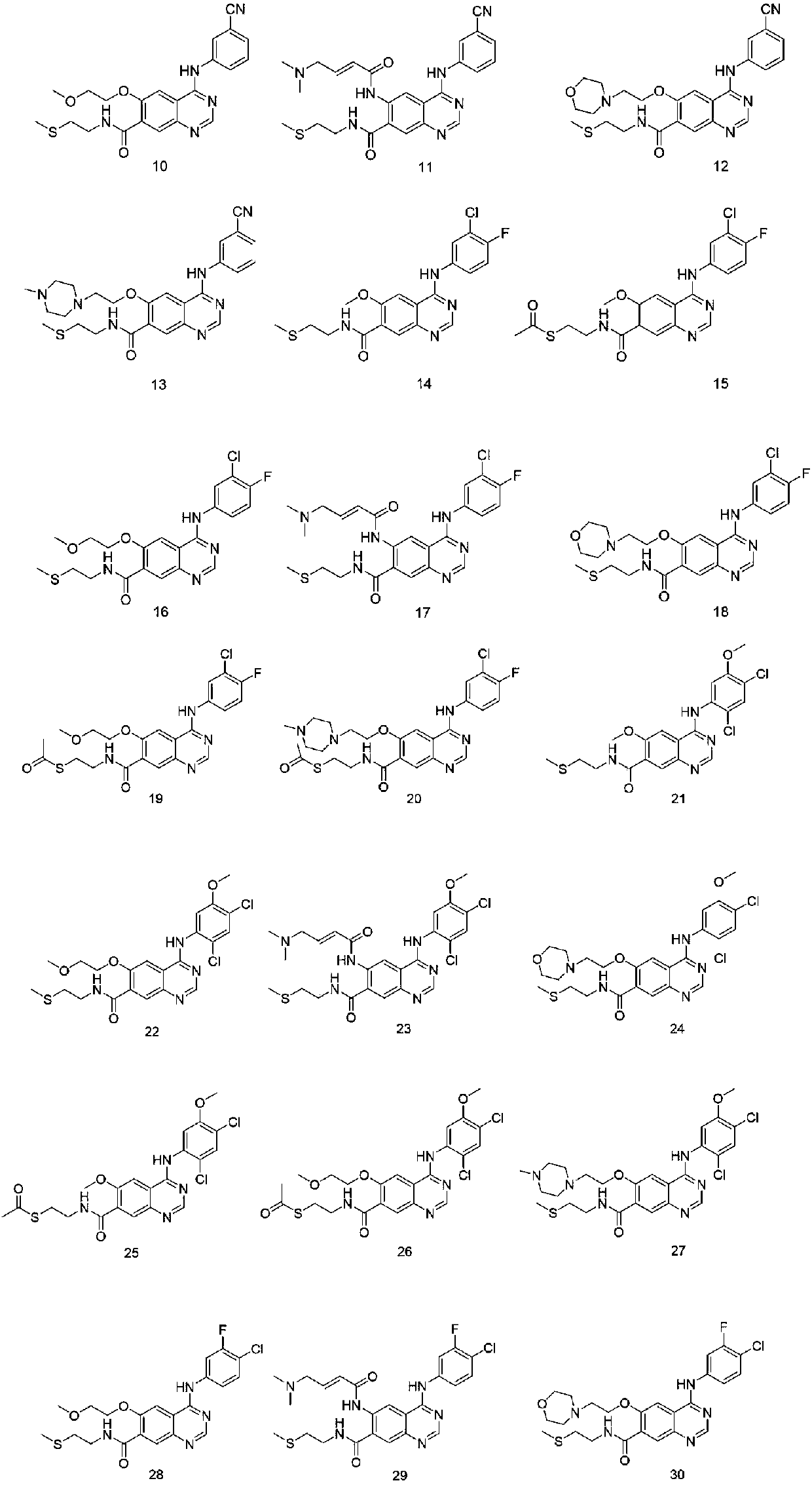
Quinazoline derivative and application thereof
A technology of quinazoline and its derivatives, which is applied in the field of chemical synthesis of drugs, can solve problems such as C797S drug-resistant mutations, and achieve significant curative effect and high in vitro inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Adopt synthetic route one to prepare 6-methoxy-4-(2-isopropylsulfonylanilide) base-7-(2-thiomethylethylamine) ketoquinazoline, the specific process is as follows:
[0046] 1) Intermediate 1, namely 6-methoxy-4-chloro-7-(2-thiomethyl ether ethylamine) ketone quinazoline (0.3g, 1mmol), was dissolved in DMF, and iodomethane ( 0.17g, 1.2mmol), anhydrous sodium carbonate (0.011g, 0.1mmol), react at room temperature for 12h. After the reaction was completed, a large amount of water was added to the reaction solution, and a large amount of solid precipitated out of the solution, which was suction filtered, and the filter cake was washed with a small amount of water and dried to obtain 0.3 g of a yellow product.
[0047] 2) Dissolve the product obtained in step 1) in DMF, add sodium hydride (0.08g, 1.8mmol) in batches under ice-cooling, react for 30min, add 2-isopropylsulfonanilide (0.23g, 1.2mmol) at 0°C ), return to room temperature and react for 2 hours, add a large amount ...
Embodiment 2
[0050] Adopt synthetic route one to prepare 6-methoxy-4-(2-isopropylsulfonylanilide) base-7-(2-thioformylethylamine) ketoquinazoline, the specific process is as follows:
[0051]1) Intermediate 1, namely 6-methoxyl-4-chloro-7-(2-thiomethyl ether ethylamine) ketone quinazoline (0.3g, 1mmol), was dissolved in DMF, and acetyl chloride ( 0.17g, 1.2mmol), anhydrous sodium carbonate (0.11g, 1mmol), react at room temperature for 12h. After the reaction was completed, a large amount of water was added to the reaction solution, and a large amount of solid precipitated out of the solution, which was filtered with suction, and the filter cake was washed with a small amount of water and dried to obtain 0.33 g of a yellow product.
[0052] 2) Dissolve the product obtained in step 1) in DMF, add sodium hydride (0.08g, 1.8mmol) in batches under ice-cooling, react for 30min, add 2-isopropylsulfonanilide (0.23g, 1.2mmol) at 0°C ), return to room temperature and react for 2 hours, add a large ...
Embodiment 3
[0055] Adopt synthetic route two to prepare 6-methoxyethoxy-4-(2-isopropylsulfonanilide) base-7-(2-thiomethyl ether ethylamine) ketone quinazoline, the specific process is as follows:
[0056] 1) Dissolve intermediate 4, namely 4,6-dichloro-7-(2-thiomethyletherethylamine)methanone quinazoline (0.6g, 2mmol) in DMF, add iodomethane (0.34g, 2.4mmol), anhydrous sodium carbonate (0.46g, 2mmol), react at room temperature for 12h. After the reaction was completed, a large amount of water was added to the reaction solution, and a large amount of solid precipitated out of the solution, which was suction filtered, and the filter cake was washed with a small amount of water and dried to obtain 0.6 g of a yellow product.
[0057] 2) Dissolve the product obtained in step 1) into DMF, add potassium carbonate (0.35g, 3mmol) and ethylene glycol methyl ether (0.5g, 3mmol), and react at 80°C for 8h. After the reaction is complete, add With a large amount of water, a yellow solid precipitated o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com