Propylene production method
A manufacturing method and technology of propylene, applied in the direction of organic chemical methods, chemical instruments and methods, double decomposition reaction to hydrocarbon production, etc., can solve the problems of difficult changes in the supply and demand balance of propylene and ethylene, and achieve the effect of high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] (Examples 1 to 6, Comparative Example 1)
[0172]
[0173] Regarding the aluminosilicates of preparation examples 1 to 5, the NH 3 -TPD and pyridine-TPD were used to measure the total acid content and the external surface acid content, respectively. The measurement was performed as follows using an automatic temperature rising desorption analyzer TP5500 manufactured by Nippon BEL Corporation.
[0174] (overall acidity)
[0175] 30 to 50 mg of aluminosilicate as a sample was dried in a helium atmosphere at 500° C. for 1 hour to desorb adsorbed substances such as organic matter and water. Thereafter, the sample was kept at 100° C. for 15 minutes under a 5 vol % ammonia / helium atmosphere to allow the sample to adsorb ammonia. Then contact with water vapor at 100°C to remove the remaining ammonia, and obtain the aluminosilicate with adsorbed ammonia. Then, the temperature of the aluminosilicate having adsorbed ammonia was raised at a rate of 10°C / min in a helium envir...
Embodiment 1~4
[0179] (Examples 1 to 4, Comparative Example 1)
[0180] During the reaction, a normal-pressure fixed-bed circulation reactor was used, and a reaction tube made of quartz with an inner diameter of 6 mm was filled with a mixture of 100 mg of aluminosilicate and 400 mg of quartz sand in Preparation Examples 1 to 5. The mixed gas of 30% by volume of ethylene and 70% by volume of nitrogen, the weight space velocity of ethylene is 0.73Hr -1 The reactor was supplied to react at 400° C. and 0.1 MPa. After the reaction started, after 2.75 hours for the unmodified catalyst (Comparative Example 1) and 1.92 hours for the modified catalyst (Examples 1-4), the products were analyzed with a gas chromatograph. The results are shown in Table 1.
Embodiment 5、6
[0182]For the reaction, a normal-pressure fixed-bed circulation reaction device was used, and a reaction tube made of quartz with an inner diameter of 6 mm was filled with a mixture of 100 mg of aluminosilicate and 400 mg of quartz sand in Preparation Example 1. The mixed gas of 30% by volume of ethylene and 70% by volume of nitrogen, the weight space velocity of ethylene is 0.36Hr -1 The reactor was supplied, and the reaction was carried out at 350° C. and 0.1 MPa. After the reaction started, the product was analyzed with a gas chromatograph after 1.92 hours and 3.17 hours. The results are shown in Table 1.
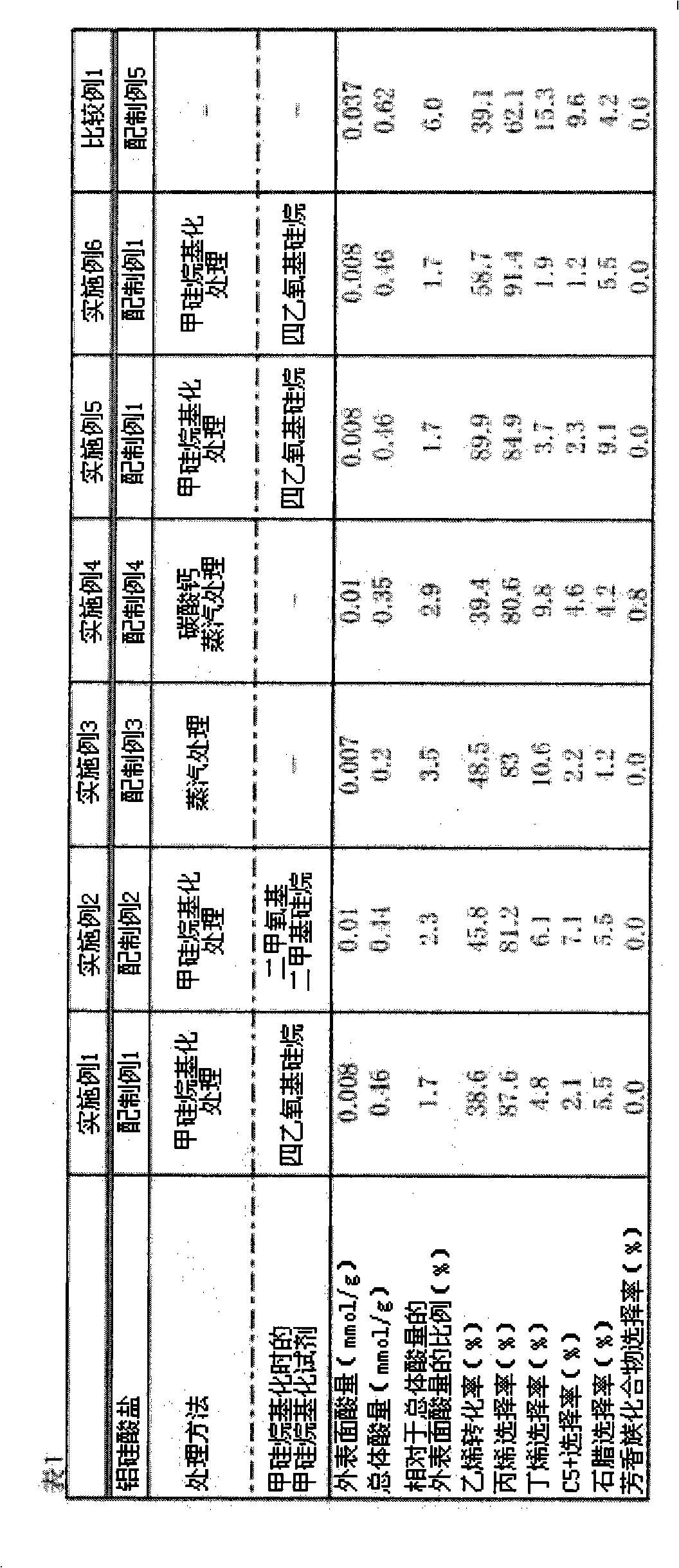
[0183] [Table 1]
[0184]
[0185] According to the result of Table 1, it can be seen that the catalysts of Examples 1-4, compared with the catalyst of Comparative Example 1, have high propylene selectivity, butene selectivity, C 5 + Selectivity, and low paraffin selectivity.
[0186] In addition, from the results in Table 1, it can be seen that the catalysts of E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com