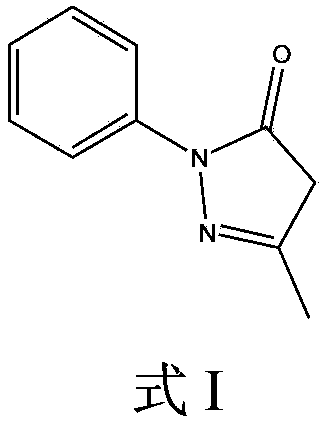
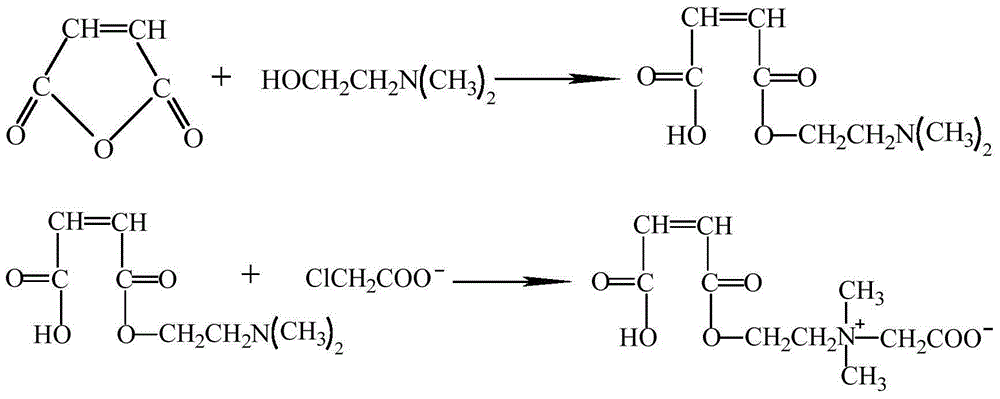
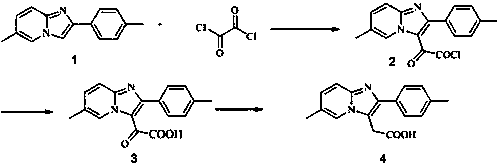
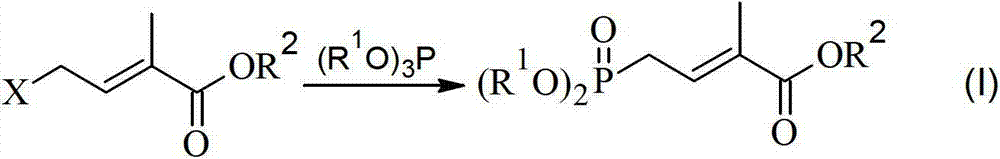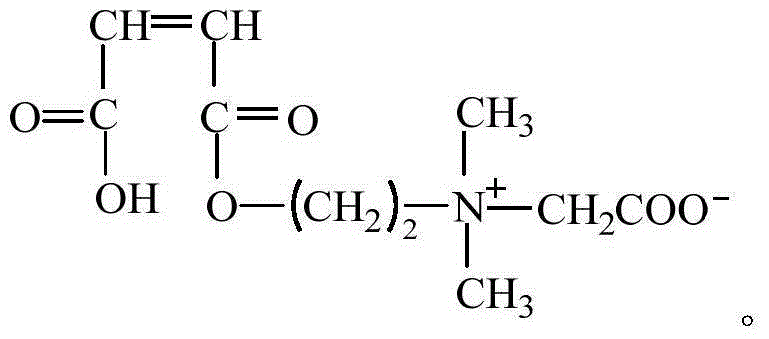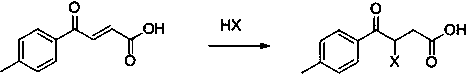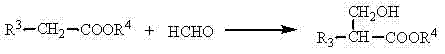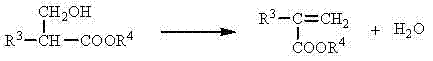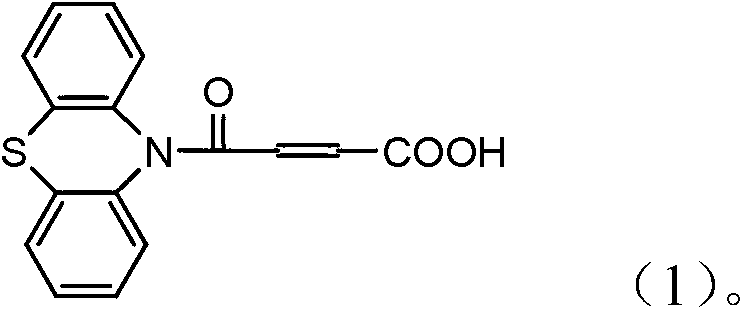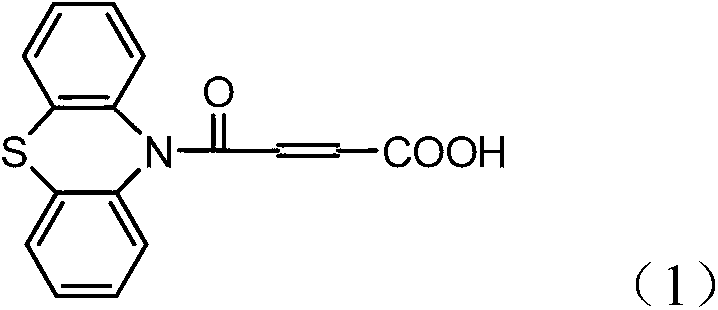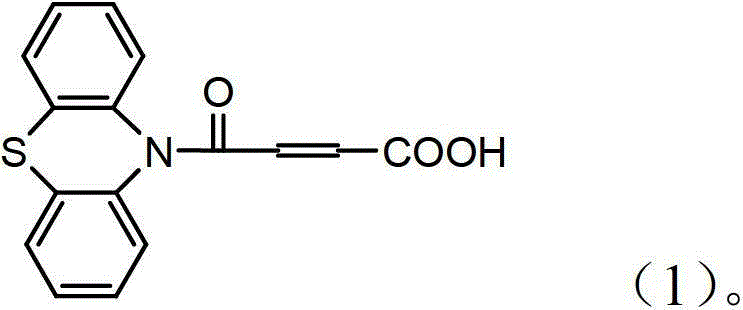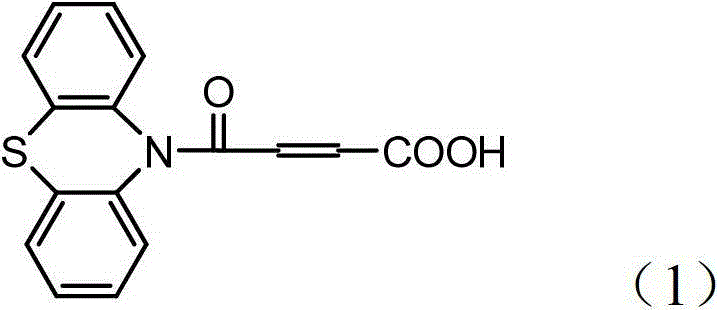Patents
Literature
41 results about "2 Butenoic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
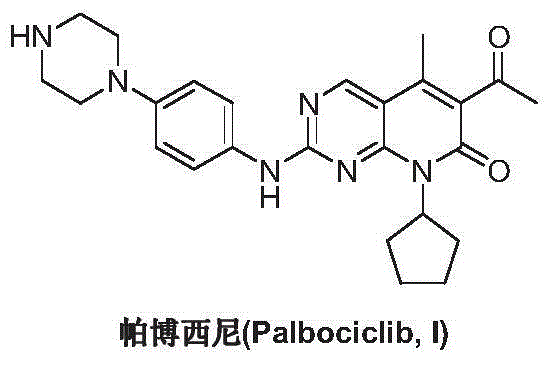
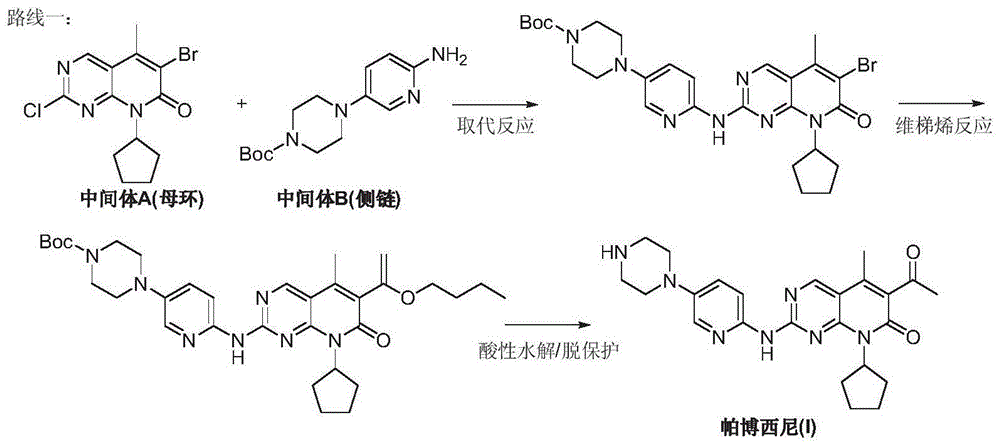
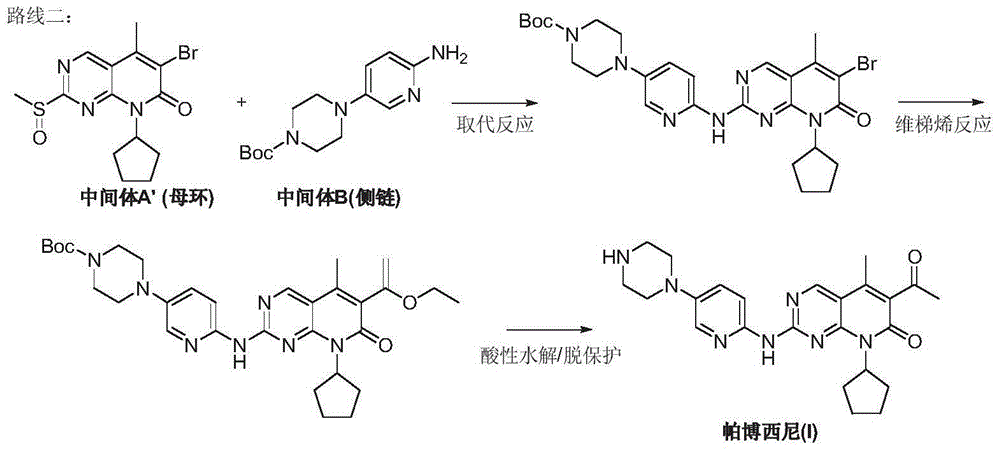
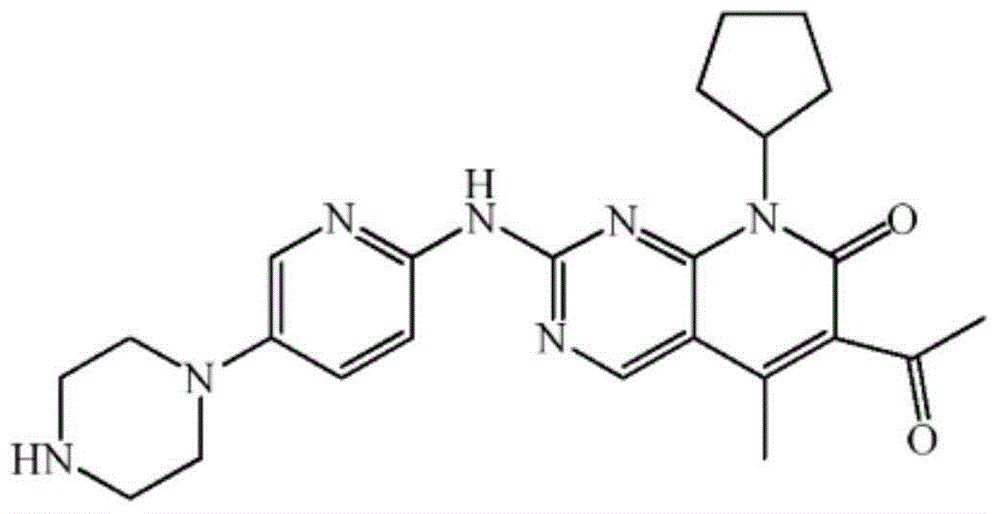
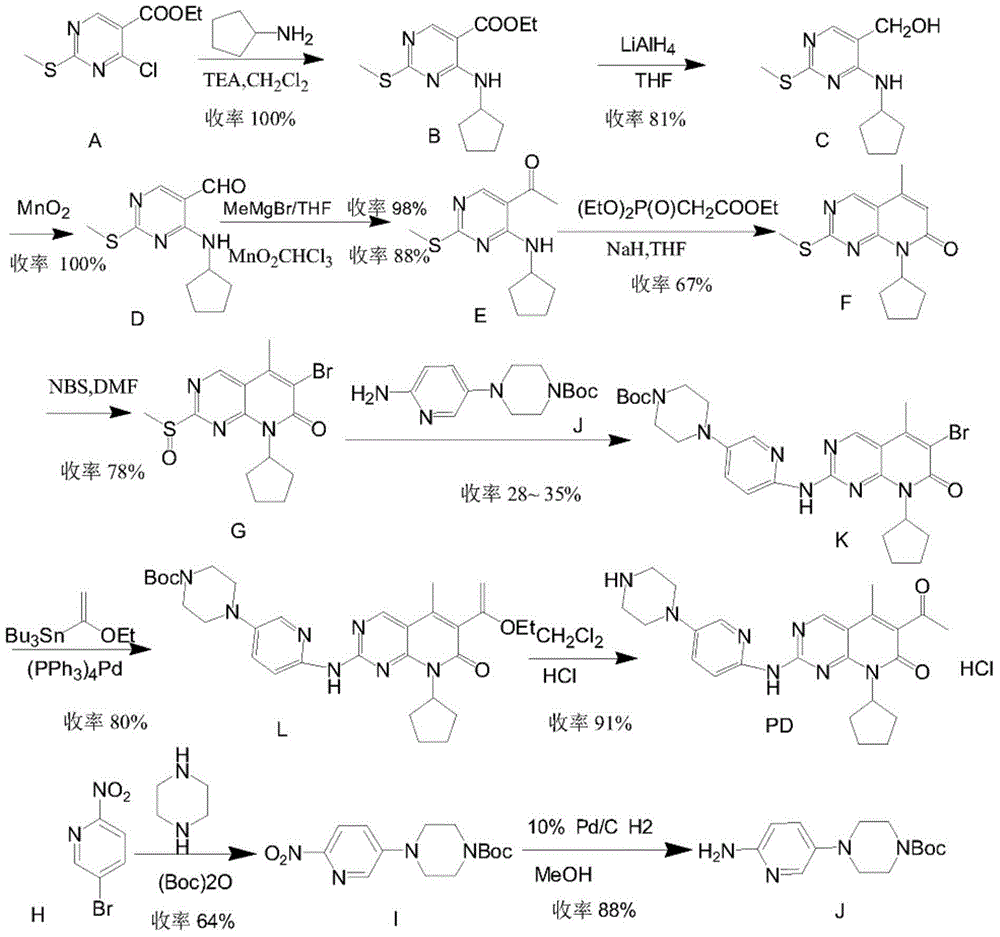
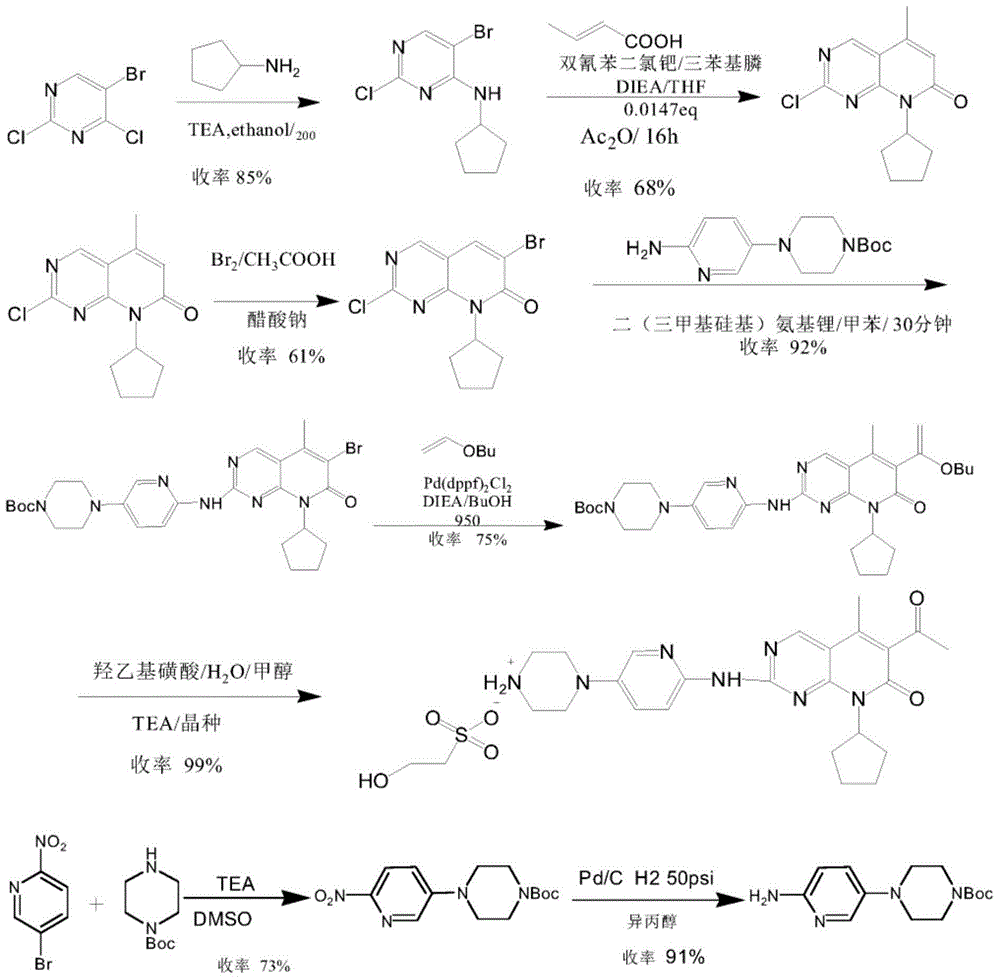
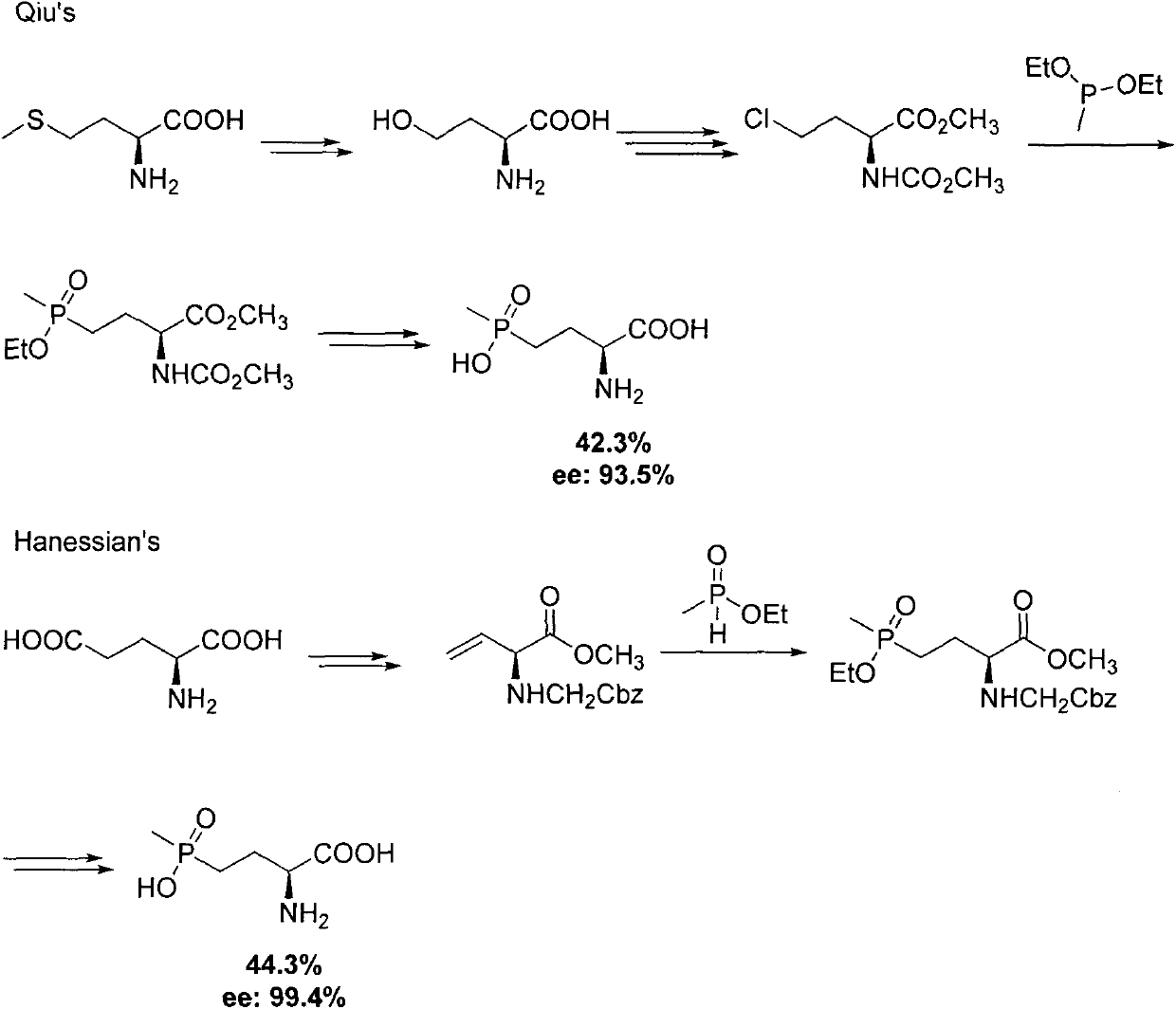
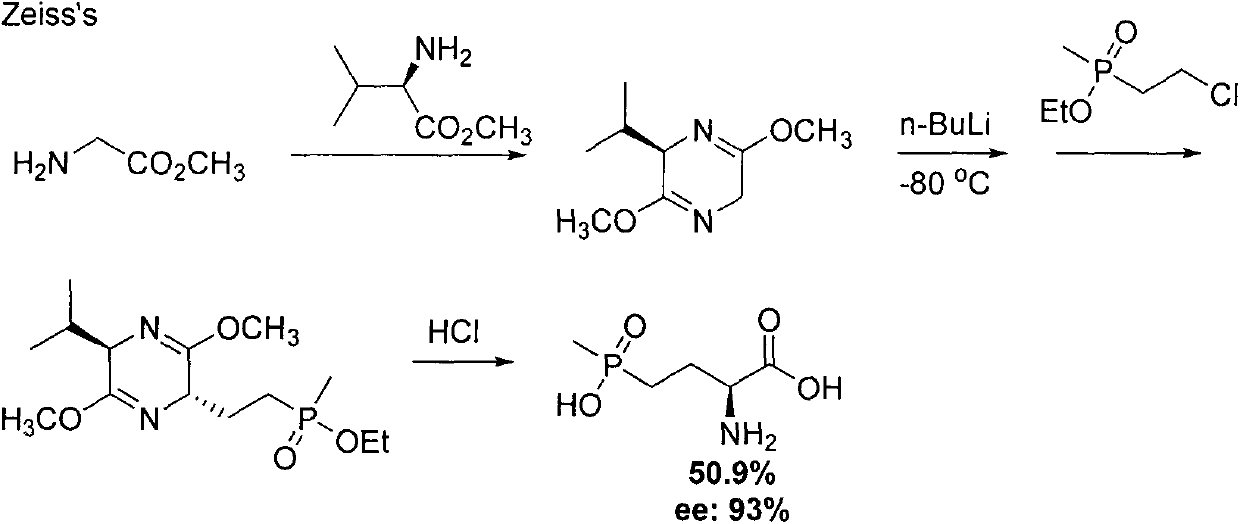
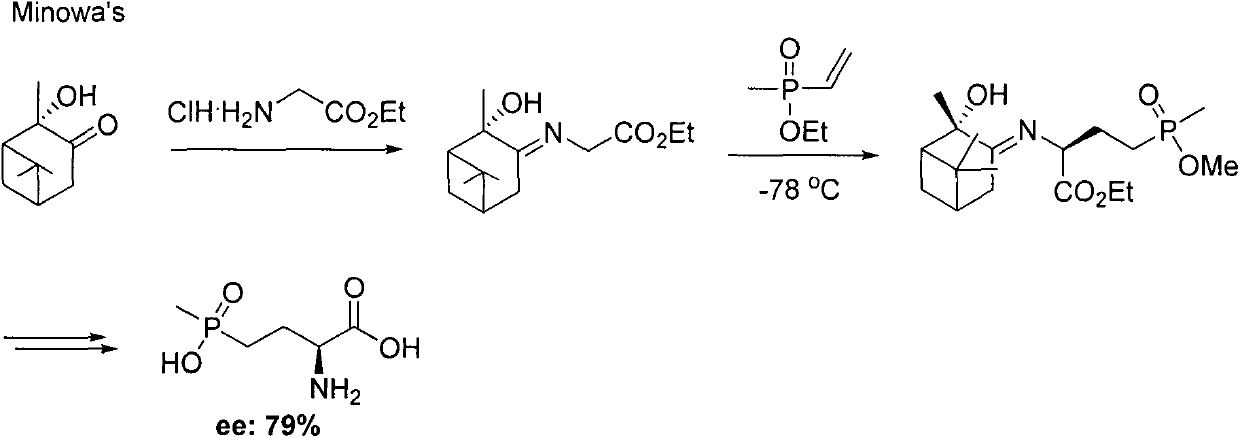
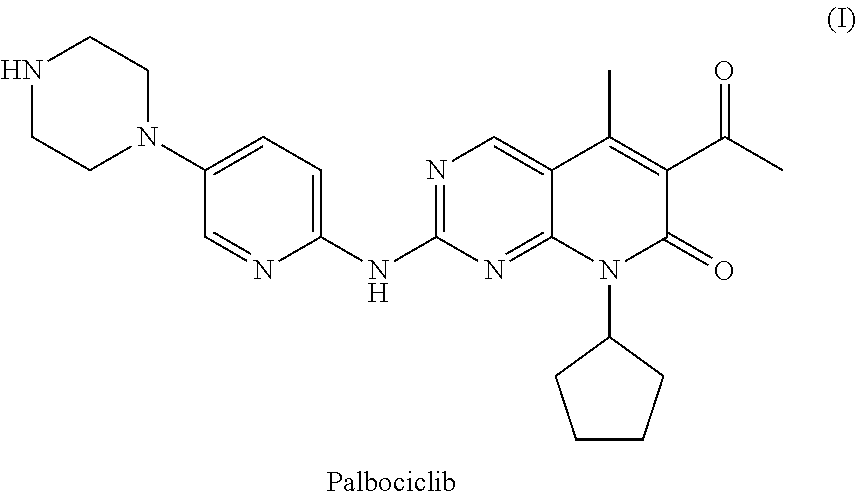
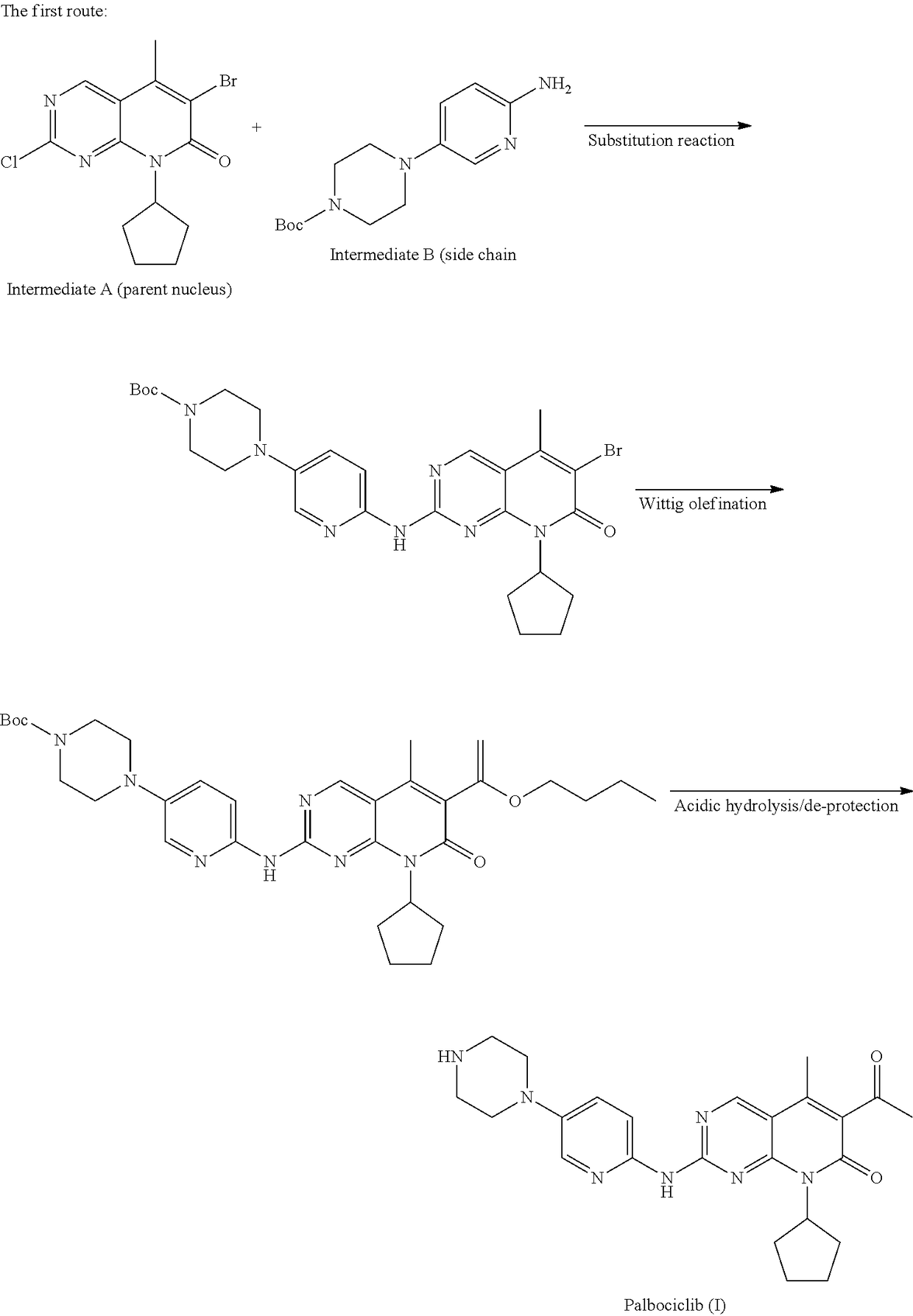
Preparation method for palbociclib
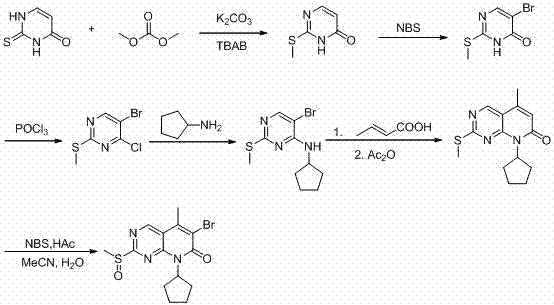
ActiveCN104447743AEase of industrial productionRaw materials are easy to getOrganic active ingredientsAmino group formation/introductionDehydrogenationKetone
The invention discloses a preparation method for palbociclib (I). The preparation method comprises the following steps: performing cyclization reaction on 2-acetyl-2-butenoic acid methyl ester and malononitrile under an alkaline condition to generate 1,4,5,6-4H-2-methoxyl-4-methyl-5-acetyl-6-oxo-3-pyridine carbonitrile (II); performing substitution reaction on the intermediate (II) and cyclopentane halide (III) under the action of an acid-binding agent to generate N-cyclopentyl-1,4,5,6-4H-2-methoxyl-4-methyl-5-acetyl-6-oxo-3-pyridine carbonitrile (IV); performing condensation reaction on the intermediate (IV) and N-[5-(1-piperazinyl)-2-piperidyl]carbamidine (V)to generate 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-5,6-dihydropyrido[2,3-d]pyrimidine-7(8H)-ketone (VI); performing dehydrogenation reaction on the intermediate (VI) and sodium selenate to generate the palbociclib (I). The preparation method for the palbociclib has the advantages that the raw material is easy to obtain, the process is simple, high efficiency and environmental protection are achieved, and the preparation method is suitable for industrial production.
Owner:铜陵王燕堂生物科技有限公司
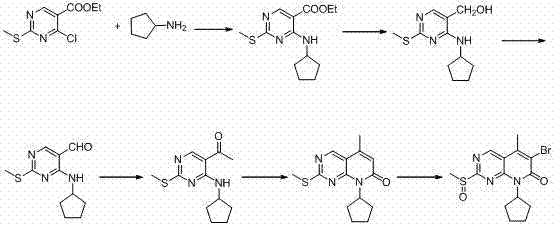
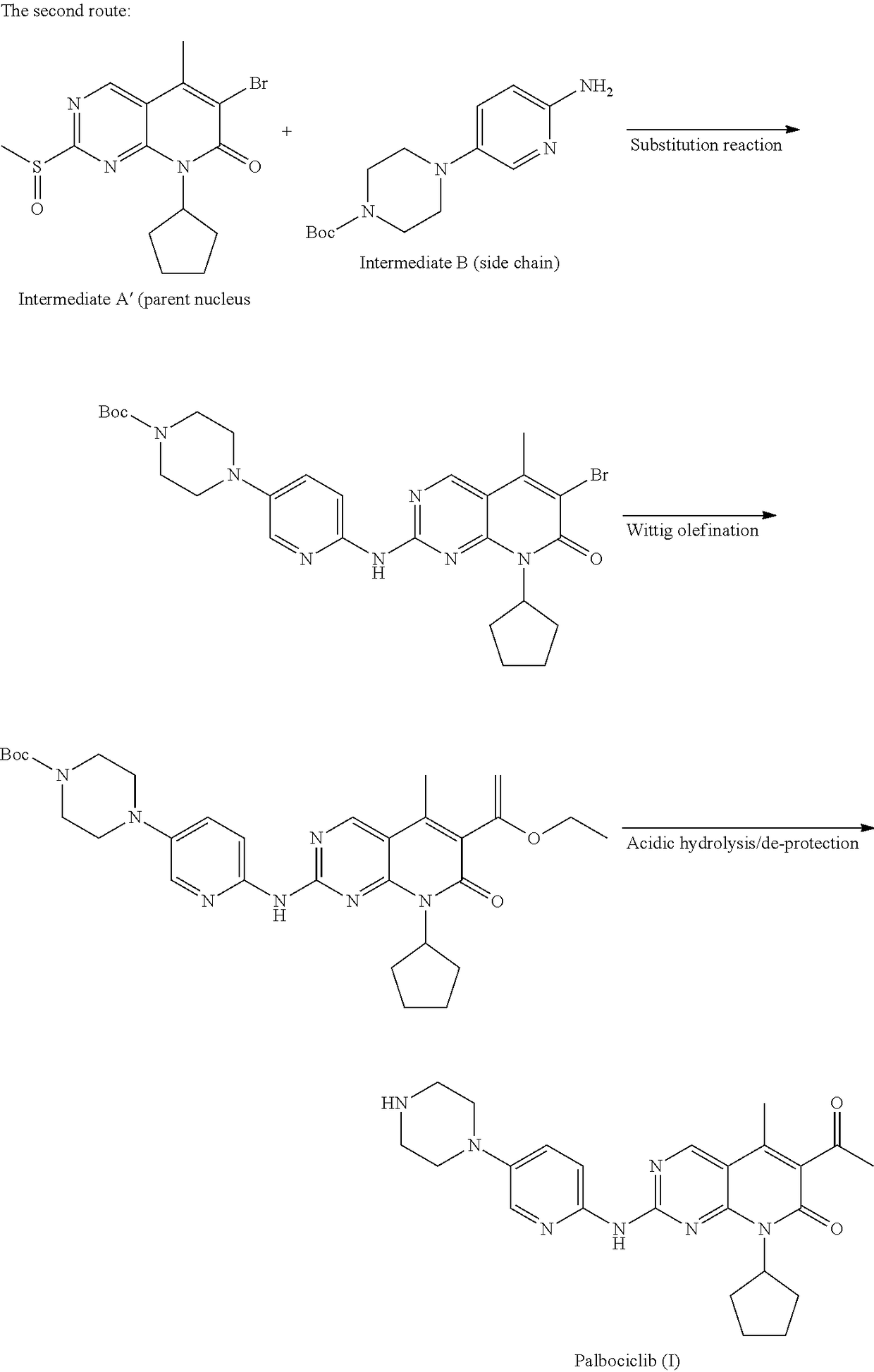
Preparation method of palbociclib
The invention belongs to the field of pharmaceutical and chemical engineering, and particularly relates to a preparation method of palbociclib. 2-acetyl-2-butenoic acid methyl ester, malononitrile and a guanidino compound III are reacted together according to an ultrasonic-microwave assisted synthesis method, and a compound IV is rapidly obtained with a high yield; then, sodium nitrite and hypophosphorous acid are subjected to a deamination reduction reaction to generate a compound V; then, the compound V and cyclopentane halide are subjected to a coupled reaction under the action of a catalyst to generate a compound VI; finally, a dehydrogenation reaction is conducted under the action of a catalyst TPND to obtain the palbociclib. The method has the advantages that reaction conditions are mild, the technological process is simple and reasonable, reaction time is short, aftertreatment is easy, product quality is high, and the yield is high.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
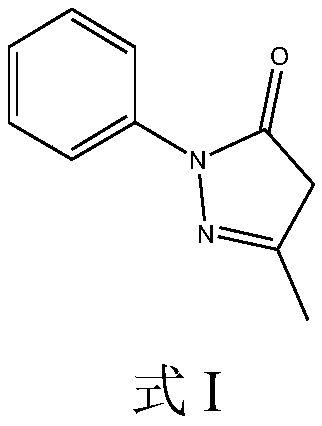
Edaravone derivative, and preparation method, detection method and application thereof
InactiveCN104098512AHigh purityEasy to prepareOrganic chemistryComponent separation2 Butenoic AcidsOrganic solvent
The invention discloses an edaravone derivative, and a preparation method, a detection method and application thereof. The invention relates to a compound with the structure shown in the specification, and a preparation method, a detection method and application thereof. The preparation method for the edaravone derivative is characterized by comprising: dissolving edaravone in an organic solvent, reacting with an aqueous solution of Hydrogen peroxide, then utilizing reversed-phase high-performance liquid chromatography to perform separation on the above edaravone reaction solution, so as to prepare (Z)-2-(3-methyl-5-oxo-1-phenyl-4,5-dihydro-1H-pyridin-4-yl)-3-[(E)-phenylazo]-2-butenoic acid. Additionally, the invention also provides the detection method for the compound. The compound can be used as a control sample of edaravone impurities, and is convenient for controlling edaravone bulk drugs and the content of the compound in correlated preparations.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD +1
Amino lyase mutant protein and coding gene and application thereof
ActiveCN108866028AHigh aminolyase activityHigh yieldGenetic engineeringFermentation2 Butenoic AcidsMutated protein
The invention discloses amino lyase mutant protein and a coding gene and application thereof. The protein is obtained by conducting mutation on at least one of 187-326 site amino acid residue shown inthe sequence 2 of the sequence table. The invention further discloses a method for utilizing the protein to prepare (R) 1-propyl(2-amino)formic acid. The protein provided by the invention has the relatively high amino lyase activity, and meanwhile can catalyze an ammonification reaction which taking trans-2-butenoic acid as a substrate to produce (R) 1-prypyl(2-amino)formic acid, the yield is high, the requirements of 100% stereoscopic selectivity is satisfied, and the protein has the very wide application prospects.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Method for producing 2-butenoic acid
ActiveCN101979371AWell mixedIncrease reaction pressureOrganic compound preparationChemical/physical/physico-chemical nozzle-type rreactors2 Butenoic AcidsOxygen
The invention provides a method for producing 2-butenoic acid, which comprises the following steps: preparing mixed feed liquid from 2-butenoic aldehyde, normal hexane and water in a weight ratio of 1:1:0.05-0.08; mounting an ejector pump on an oxidation reaction kettle for spraying the mixed feed liquid into the oxidation reaction kettle; introducing oxygen into the oxidation reaction kettle to perform a reaction under a condition that the reaction pressure in the oxidation reaction kettle is 0.1 to 0.6MPa and a condition that the temperature is 25 to 35 DEG C to obtain oxidation reaction solution; distilling the oxidation reaction, distilling the remaining 2-butenoic aldehyde under a reduced pressure, recovering the 2-butenoic aldehyde, distilling the remaining normal hexane at normal pressure and obtaining filtrate; and cooling the filtrate to -5 DEG C to 5 DEG C, precipitating, crystallizing and filtering and drying the crystals to obtain the 2-butenoic acid product. The production method has the advantages that: the reaction speed is high; the product yield is high; the energy consumption is small; and the quality of the product obtained is high.
Owner:HENAN ZAITI BIO TECH
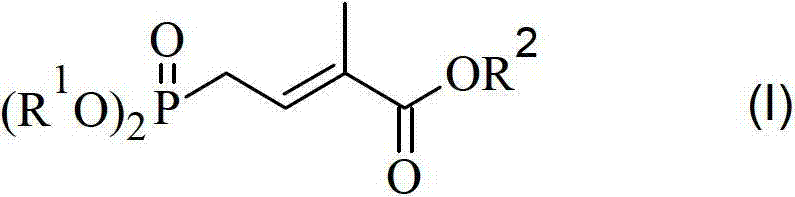
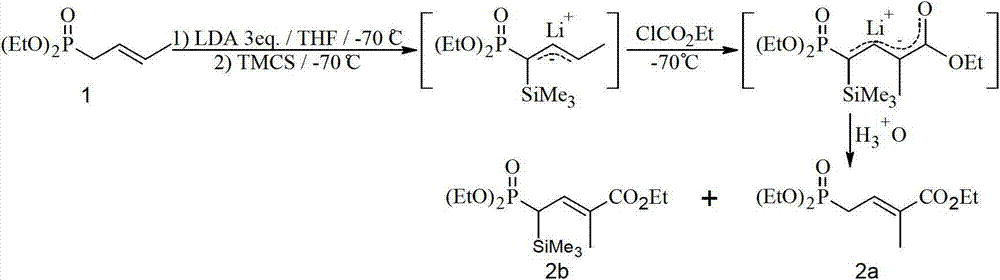
Method for preparing 4-bialkoxy-phosphono-2-methyl-2-butenoic acid alkyl ester
ActiveCN103113404AConvenient sourceReliable responseGroup 5/15 element organic compounds2 Butenoic Acids2-Methyl-2-butene
The invention discloses a method for preparing 4-bialkoxy-phosphono-2-methyl-2-butenoic acid alkyl ester. According to the method, a compound shown in a formula A and a compound shown in a formula B have reaction to generate a compound having a general formula I. The method has the advantages that raw materials are easily available sources, reaction is stable and reliable, reaction conditions are mild, and environment-friendliness effect is achieved, and the method is very suitable for industrial production.
Owner:广州巨元生化有限公司
Concrete mud resisting agent based on counter-insert layer design and preparing method thereof
The invention relates to a concrete mud resisting agent based on counter-insert layer design and a preparing method. The method comprises the following steps that micro-molecule monomers, 2-butenoic acid, sodium methallyl sulfonate and hydroxyethyl methacrylate are stirred, dissolved and kept at the temperature of 70 DEG C-75 DEG C to obtain a uniform solution, then an oxidizing agent is added, and the mixture is stirred to obtain a uniform solution; a reducing agent and chain transfer agent mixture is added in a one-time mode, the mixture continues to be stirred for 2-4 h, pH is adjusted to be 8-9, and a proper amount of deionized water is added, so that the concrete mud resisting agent based on the counter-insert layer design is obtained. The method has the advantages that the prepared concrete mud resisting agent is provided with positive-charge functional group monomers, has a good ability to be adsorbed by montmorillonite clay preferentially, and can well stop polycarboxylate superplasticizer from being adsorbed between montmorillonite layers; the concrete mud resisting agent is high in adaptability, initial slump of concrete is increased, and the concrete has good workability.
Owner:WUHAN UNIV OF TECH
Method for producing 2-butenoic acid
ActiveCN101979371BWell mixedIncrease reaction pressureOrganic compound preparationChemical/physical/physico-chemical nozzle-type rreactors2 Butenoic AcidsOxygen
The invention provides a method for producing 2-butenoic acid, which comprises the following steps: preparing mixed feed liquid from 2-butenoic aldehyde, normal hexane and water in a weight ratio of 1:1:0.05-0.08; mounting an ejector pump on an oxidation reaction kettle for spraying the mixed feed liquid into the oxidation reaction kettle; introducing oxygen into the oxidation reaction kettle to perform a reaction under a condition that the reaction pressure in the oxidation reaction kettle is 0.1 to 0.6MPa and a condition that the temperature is 25 to 35 DEG C to obtain oxidation reaction solution; distilling the oxidation reaction, distilling the remaining 2-butenoic aldehyde under a reduced pressure, recovering the 2-butenoic aldehyde, distilling the remaining normal hexane at normal pressure and obtaining filtrate; and cooling the filtrate to -5 DEG C to 5 DEG C, precipitating, crystallizing and filtering and drying the crystals to obtain the 2-butenoic acid product. The production method has the advantages that: the reaction speed is high; the product yield is high; the energy consumption is small; and the quality of the product obtained is high.
Owner:HENAN ZAITI BIO TECH
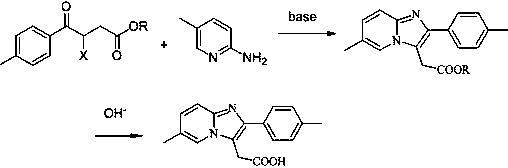
Preparation method of zolpidic acid
The invention discloses a synthesis method of zolpidic acid. Different from the prior art, the synthesis method disclosed by the invention comprises the following steps: firstly performing a Friedel-Crafts reaction of toluene and maleic anhydride to obtain 4-oxo-4-(4-methylphenyl)-2-butenoic acid, then performing addition with hydrohalogen acid to obtain 3-halogenated-4-oxo-4-(4-methylphenyl)-butyric acid, performing esterification and cyclization to obtain 2-(6-methyl-2-p-methylbenzimidazole[1,2-alpha]pyridine-3-yl)acetate, and performing hydrolysis and acidification to obtain zolpidic acid.The method disclosed by the invention can obtain zolpiotan acid with a high purity, and the whole synthetic route has the advantages of few steps, high yield, low cost and few impurities, and is suitable for industrial production.
Owner:烟台万润药业有限公司
Method for synthesizing pesticide intermediate 2-amido-4-(O-alkylmethylphosphonyl)-2-butenoic acid and its ester
The invention belongs to the technical field of pesticide intermediates for synthesizing herbicides and particularly relates to a method for synthesizing a pesticide intermediate 2-amido-4-(O-alkylmethylphosphonyl)-2-butenoic acid and its ester. The method comprises that a compound VII and a compound VI undergo an Arbuzov reaction to produce a compound V, the compound V is hydrolyzed through an acid to produce a compound IV, the compound IV and a compound III undergo a reaction under action of a condensation reagent and a catalyst to produce a compound II and the compound II is hydrolyzed into a compound I. Compared with the existing synthesis route, the method has simple processes, high atom economy and high stereoselectivity, is environmentally friendly, realizes a low cost and has an industrial prospect. In the formula, R1 represents C1-4 alkyl; R2 and R3 represent CnH2n+1 and n is 1, 2, 3 or 4, or R2 or R3 represents -CmH2m- and m is 2 or 3; R4 represents C1-4 alkyl or C6-10 aryl; R5 represents hydrogen or C1-4 alkyl; and X represents chlorine or bromine.
Owner:NANJING UNIV OF TECH
Measurement method of volatile fatty acid in tobacco leaf
ActiveCN109061005AComprehensive resultsThe measurement result is accurateComponent separationVolatile fatty acidsAcetic acid ear
The invention relates to a measurement method of volatile fatty acid in tobacco leaf. The measurement method comprises the following steps: 1) adding dichloromethane in a tobacco leaf sample to perform extraction, separating to obtain extract liquor; and 2) adding derivatization reagent in the extract liquor, performing gas chromatograph-tandem mass spectrometer after the derivatization reaction.The measurement method of the volatile fatty acid in the tobacco leaf provided by the invention, the tobacco leaf sample is extracted by using the dichloromethane at the normal temperature, the C1-C10volatile fatty acid can be effectively extracted, and the detection analysis is performed after the derivatization reaction; the whole measurement process is less in step and compact in flow, the loss of the volatile component is reduced, the measurement of formic acid, acetic acid, butyric acid, 2-methylbutyric acid, 2-butenoic acid, isovaleric acid, valeric acid, senecioic acid, tiglic acid, 3-methylvaleric acid, 4-methylvaleric acid and like seventeen volatile fatty acids; a measurement result on the volatile fatty acid in the tobacco leaf is more comprehensive and accurate.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Method of efficiently preparing alpha,beta-unsaturated carboxylic acids or esters
InactiveCN104262142AWide variety of sourcesEasy to manufactureOrganic compound preparationCarboxylic acid esters preparationPetrochemicalCis-Butenedioic Acid
The invention discloses a method of efficiently preparing alpha,beta-unsaturated carboxylic acids or esters, and belongs to the technical field of industrial catalysis in petrochemical engineering. Formaldehyde or sources thereof and carboxylic acids or esters are subjected to a gas-solid phase reaction under the existence of a gamma-Al2O3 catalyst supported by a K-sourced or Cs-sourced precursor, and under the existence of an alcohol as appropriate. The carboxylic acids or esters which are reactants have a general formula that is R3-CH2-COOR4, wherein the R4 is hydrogen or alkyl, and the R3 is hydrogen, alkyl or aryl. The prepared alpha,beta-unsaturated carboxylic acids or esters comprise acrylic acid, alkyl acrylic acid, 2-butenoic acid, cyclohexene-1-carboxylic acid, maleic acid, itaconic acid, fumaric acid, and alkyl esters and methylene-substituted lactones of the acrylic acid, the alkyl acrylic acid, the 2-butenoic acid, the cyclohexene-1-carboxylic acid, the maleic acid, the itaconic acid and the fumaric acid. The sources of the formaldehyde comprise trioxymethylene, formalin and methylal. The method has advantages of high yield, high selectivity, wide sources of catalyst supporters, low cost, simple and convenient catalyst preparation processes, and the like.
Owner:CHANGZHOU UNIV
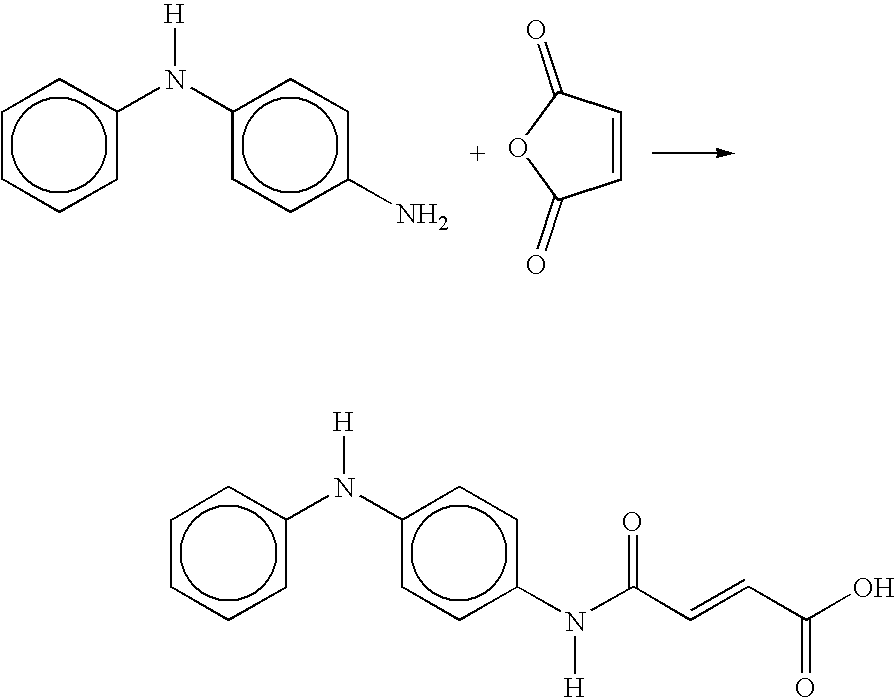
Process for the preparation of 4-OXO-4-((4-(phenylamino)phenyl)amino)-2-butenoic acid
InactiveUS20060089511A1Good dispersionEasy to handleOrganic compound preparationCarboxylic acid amides preparation2 Butenoic AcidsOrganic solvent
A process for the preparation of 4-oxo-4-((4-(phenylamino)phenyl)amino)-2-butenoic acid includes reacting p-aminodiphenyl amine and maleic anhydride in a reaction first mixture containing a volatile organic solvent under suitable reaction conditions to provide a second mixture containing 4-oxo-4-((4-(phenylamino)phenyl)amino)-2-butenoic acid product; subjecting the second mixture to vacuum distillation to remove the volatile organic solvent while simultaneously adding diluent oil to produce a dispersion of 4-oxo-4-((4-(phenylamino)phenyl)amino)-2-butenoic acid in the diluent oil.
Owner:CROMPTON CORP
Preparation method of key intermediate of palbociclib
The invention discloses a preparation method of a key intermediate of palbociclib, namely 6-bromine-2-methylsulfinyl-8-cyclopentyl-5-methyl pyridino (2,3-d) pyrimidine-7(8H)-ketone. The preparation method comprises the following steps: taking deracil as a starting material, performing methylating, chlorinating and brominating to synthesize 5-bromine-4-chlorine-2-methylthiopyrimidine, then alkylating with cyclopentylamine, conducting a heck reaction with 2-butenoic acid, then conducting an intramolecular acylation reaction to synthesize 2-methylmercapto-8-cyclopentyl-5-methyl pyridino (2,3-d) pyrimidine-7(8H)-ketone, and finally reacting with NBS to prepare the 6-bromine-2-methylsulfinyl-8-cyclopentyl-5-methyl pyridino (2,3-d) pyrimidine-7(8H)-ketone. Steps of the preparation process are short, no dangerous process is used, the operation is simple and convenient, the cost of the raw materials is low, and the use requirement of people is met.
Owner:上海微巨实业有限公司
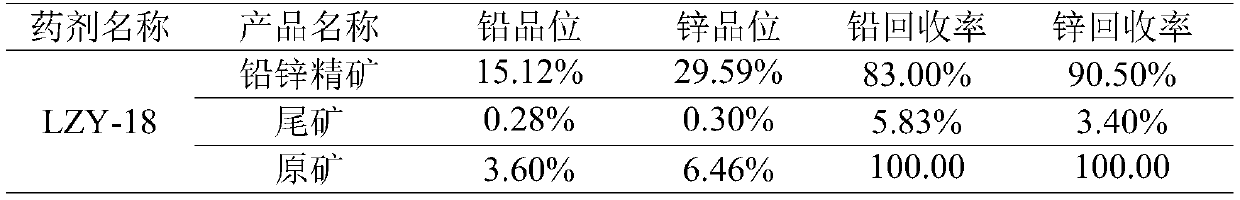
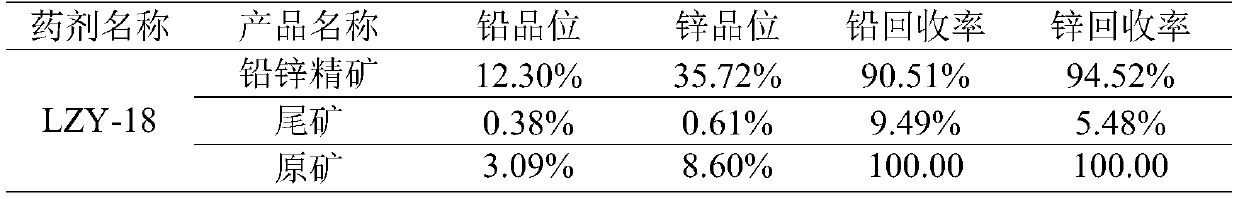
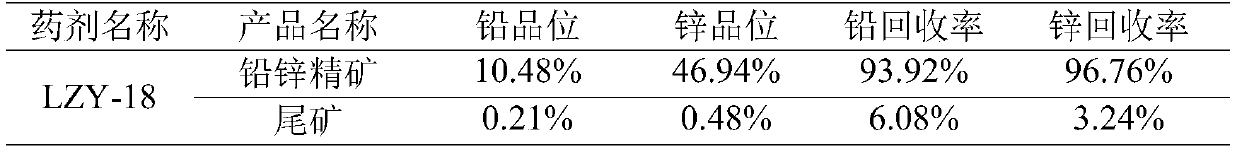
Beneficiation reagent for flotation of lead and zinc from lead-zinc oxide ore and preparation method thereof
The invention discloses a beneficiation reagent for flotation of lead and zinc from lead-zinc oxide ore and a preparation method thereof. The beneficiation reagent is prepared from the following raw materials in parts by weight: 15-25 parts of diesel oil, 10-20 parts of sodium carbonate, 20-30 parts of sodium sulfide, 40-50 parts of 3, 3-dimethylbutyric acid, 13-18 parts of sulfur, 15-30 parts ofesterified xanthate, 25-45 parts of 3-amino-2 butenoic acid, 4-5 parts of quick lime and 20-24 parts of an activating agent. The beneficiation reagent has the characteristics of high separation efficiency, high yield, low consumption, simple and reliable process flow, easiness in operation and the like, is suitable for beneficiation application of the re-vulcanized and oxidized mixed lead-zinc ore, and can bring good economic benefits to the lead-zinc ore.
Owner:NORTHWEST RES INST OF MINING & METALLURGY INST
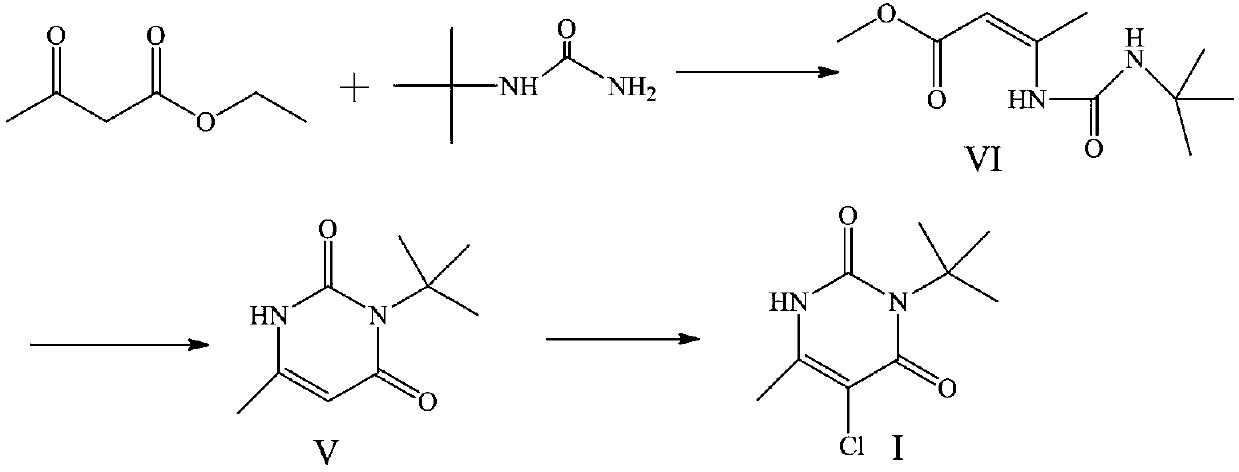
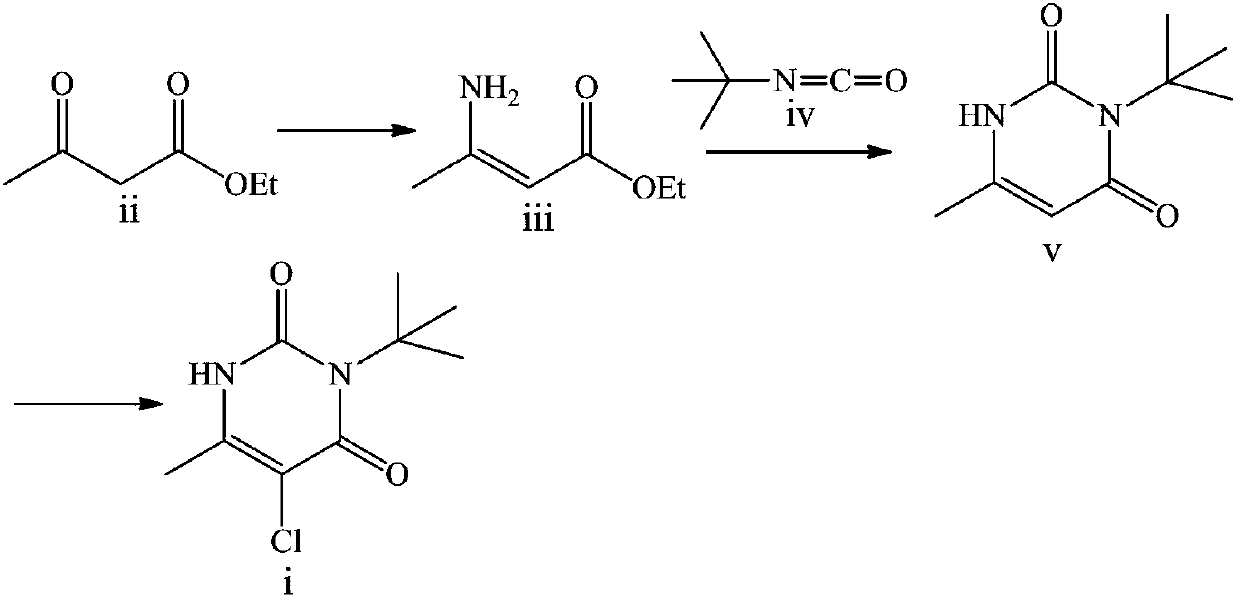
Preparation method of herbicide 3-tert-butyl-5-chloro-6-methyluracil
InactiveCN109651266AAvoid serious, low-yield defectsHigh yieldOrganic chemistryAcetic acid2 Butenoic Acids
The invention discloses a preparation method of herbicide 3-tert-butyl-5-chloro-6-methyluracil. The method comprises the steps that a compound acetoacetic ester is taken as a raw material to react with an ammonia source to generate 3-amino-2-butenoic acid ethyl ester, the 3-amino-2-butenoic acid ethyl ester is reacted with tert-butylisocyanate to generate an intermediate, and the 3-tert-butyl-5-chloro-6-methyluracil is obtained after chlorination is conducted on the intermediate. The method has the advantages that the obtaining of the raw material is easy, the technology is brief, the method is economical and environmentally friendly, and the method is suitable for industrial production.
Owner:ANHUI THERAPY PHARMA CO LTD
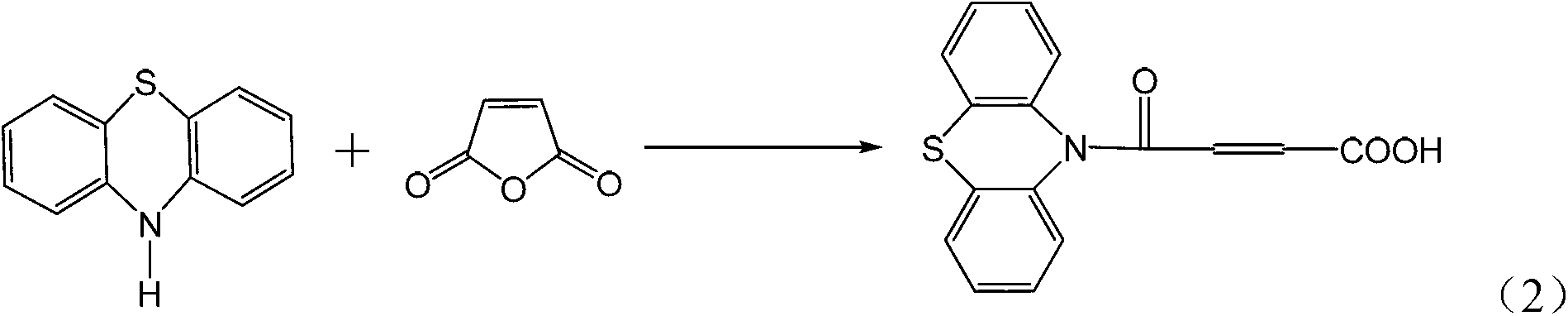
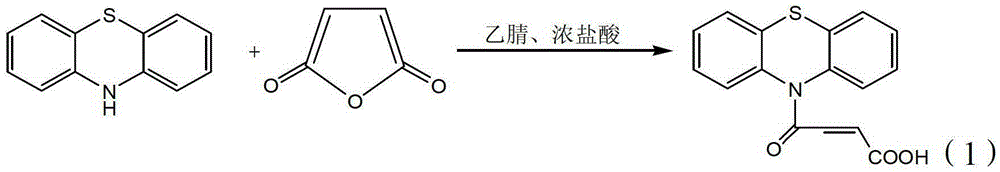
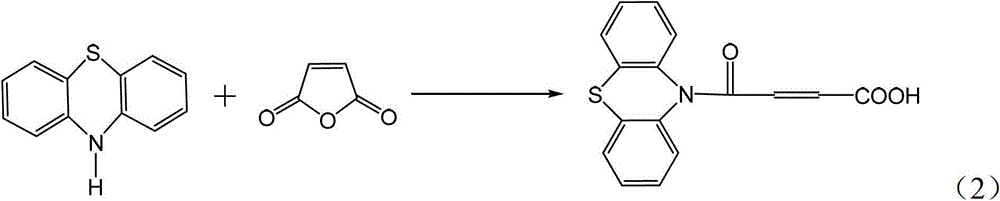
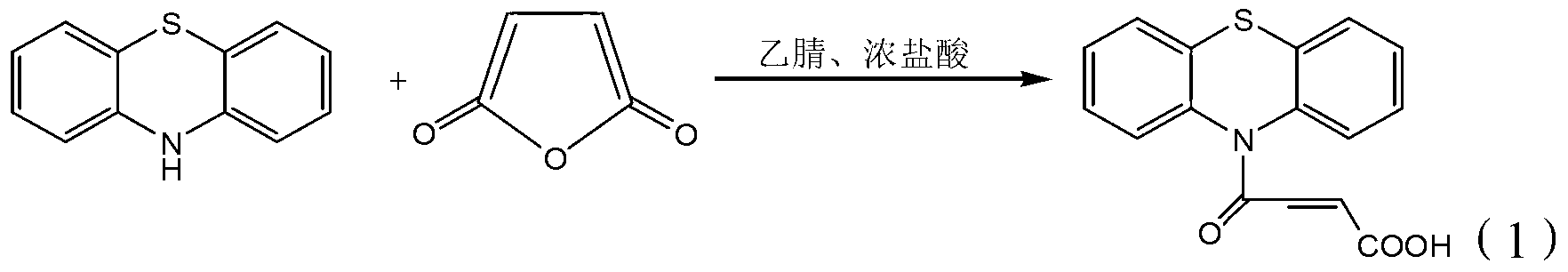
4-(N-phenthiazine-)-carbonyl-2-butenoic acid and preparation method thereof
InactiveCN103073523ASimple and fast operationReaction is easy to controlOrganic chemistry2 Butenoic AcidsFiltration
The invention discloses 4-(N-phenthiazine-)-carbonyl-2-butenoic acid and a preparation method thereof. A structural formula of 4-(N-phenthiazine-)-carbonyl-2-butenoic acid is as shown in the specification. The preparation method of 4-(N-phenthiazine-)-carbonyl-2-butenoic acid comprises the steps that A mol phenthiazine and B mol maleic anhydride are added to a reaction vessel; then C ml DMF (Dimethyl Formamide) is added; reaction liquid is obtained; the reaction liquid is heated to 100-155 DEG C on a condition of stirring and subjected to heat preservation; TLC (thin layer chromatography) is used for monitoring a reaction process; the reaction liquid is stirred continuously at 100-155 DEG C for 0.5h after raw material points are disappeared; a mixture is obtained, wherein the ratio of A to B to C is equal to 1:(1.0-1.3)-(400-800); after the mixture is cooled to a room temperature, the mixture is poured into water, subjected to suction filtration, washed and dried; and 4-(N-phenthiazine-)-carbonyl-2-butenoic acid is obtained. The method is easy and simple to operate; the reaction process is easy to control; post treatment is simple; and the productivity is high.
Owner:SHAANXI UNIV OF SCI & TECH
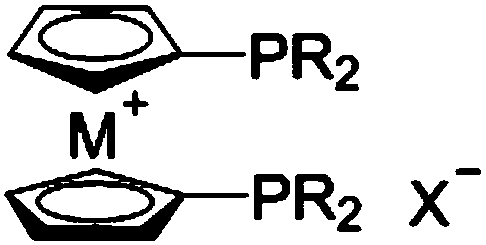
Catalyst composition and preparation method of 2-butenoic acid
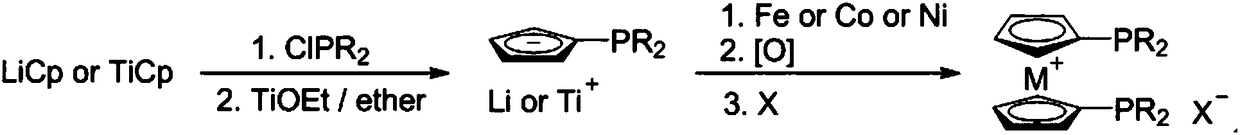
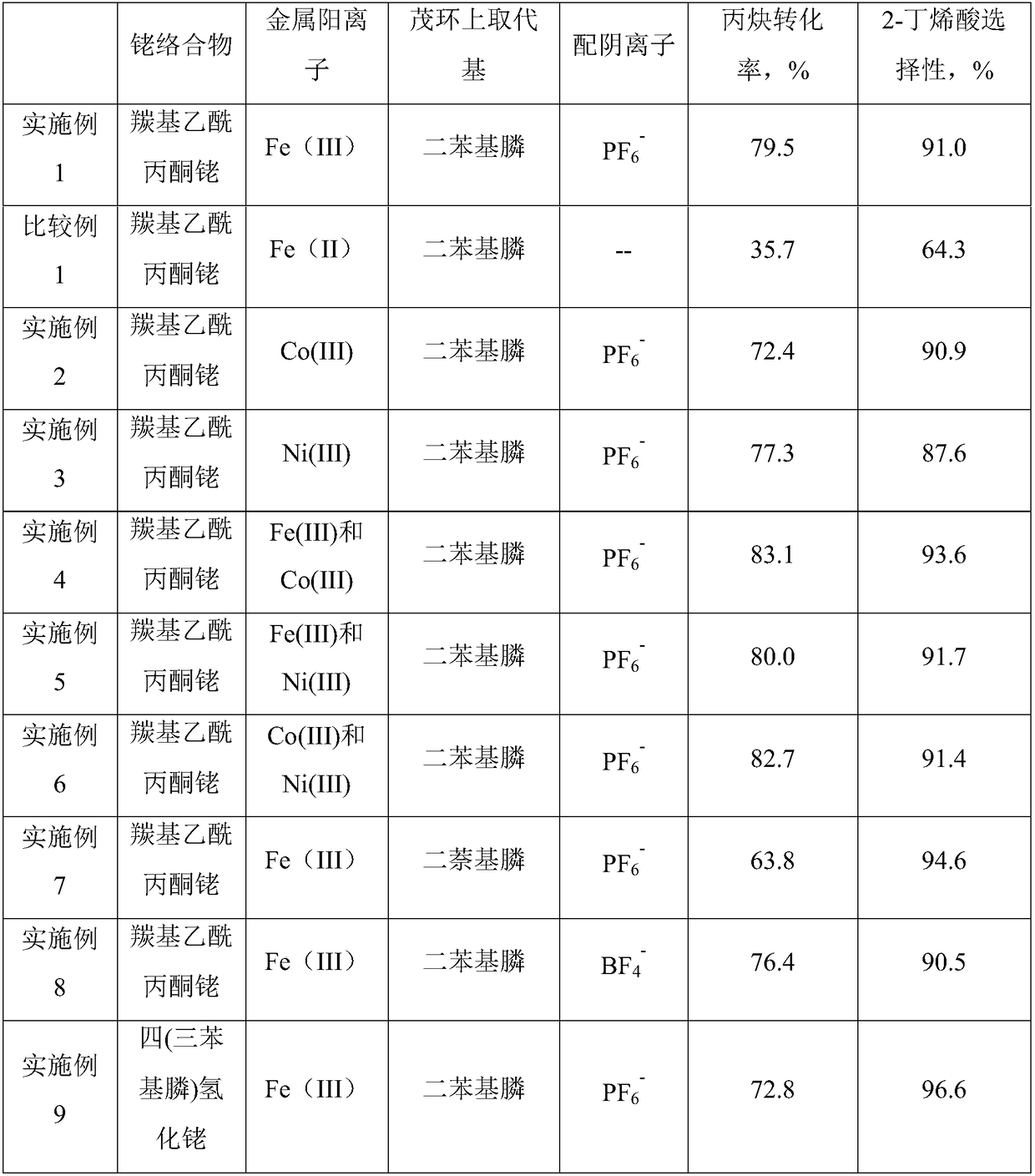
ActiveCN109092366AImprove conversion rateHigh selectivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactions2 Butenoic AcidsDiphosphines
The invention relates to a catalyst composition and a preparation method of 2-butenoic acid, and mainly solves the problems that in the prior art, the propine conversion rate is low, and the selectivity of 2-butenoic acid is low. According to the technical scheme, the catalyst composition includes a rhodium complex and a high-valence metallocene cation diphosphine compound. By means of the catalyst composition, the technical problems are well solved, and the catalyst composition can be used in industrial production of 2-butenoic acid.
Owner:CHINA PETROLEUM & CHEM CORP +1
A method for preparing 4-(n-phenothiazine-)-carbonyl-2-butenoic acid
ActiveCN103232412BImprove catalytic performanceSimple and fast operationOrganic chemistryDistillationSolvent
The invention relates to a method for preparing 4-(N-phenothiazine)-carbonyl-2-butenoic acid. The method comprises the following steps: adding A mol of phenothiazine and B mol of maleic anhydride into a reaction container, and then adding acetonitrile; dripping concentrated hydrochloric acid into the reaction container while stirring to get a reaction solution, increasing the temperature of the reaction solution from room temperature to 40-80 DEG C, and performing reaction for 1-2.5h while stirring to get a mixed solution, wherein A: B=2.5: (2.5-3.0); performing reduced-pressure distillation on the mixed solution to remove acetonitrile to get a concentrated solution, wherein the volume of acetonitrile in the concentrated solution is 15-20% of the volume of acetonitrile in the mixed solution; and cooling the concentrated solution to room temperature, then adding water into the concentrated solution, uniformly stirring, then performing suction filtration, washing a filter cake with water, and drying to get 4-(N-phenothiazine)-carbonyl-2-butenoic acid. According to the method disclosed by the invention, acetonitrile is adopted as a solvent, concentrated hydrochloric acid is taken as a catalyst, and the method has the advantages of simplicity and convenience in operation, short reaction time, mild conditions, simple post-treatment, low price and easiness in obtaining of raw materials and high yield.
Owner:SHAANXI UNIV OF SCI & TECH
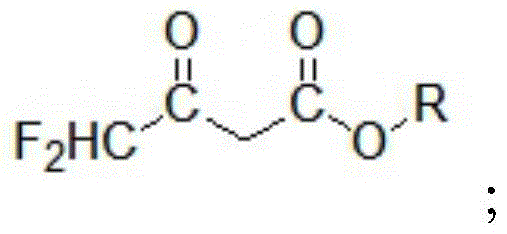
A kind of preparation method of 4,4-difluoroacetoacetate alkyl ester
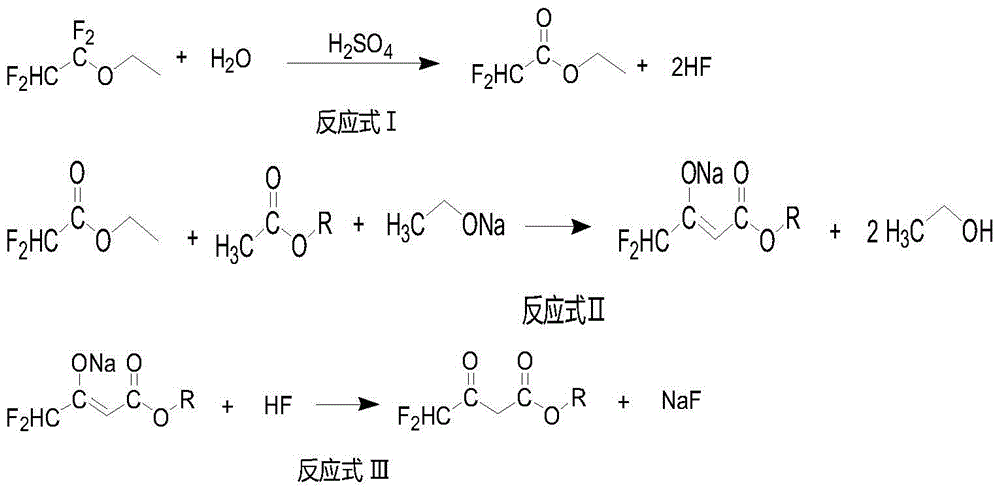
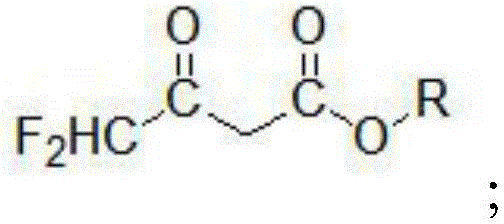
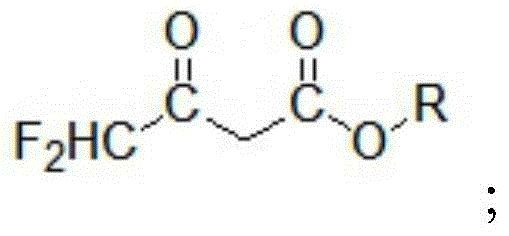
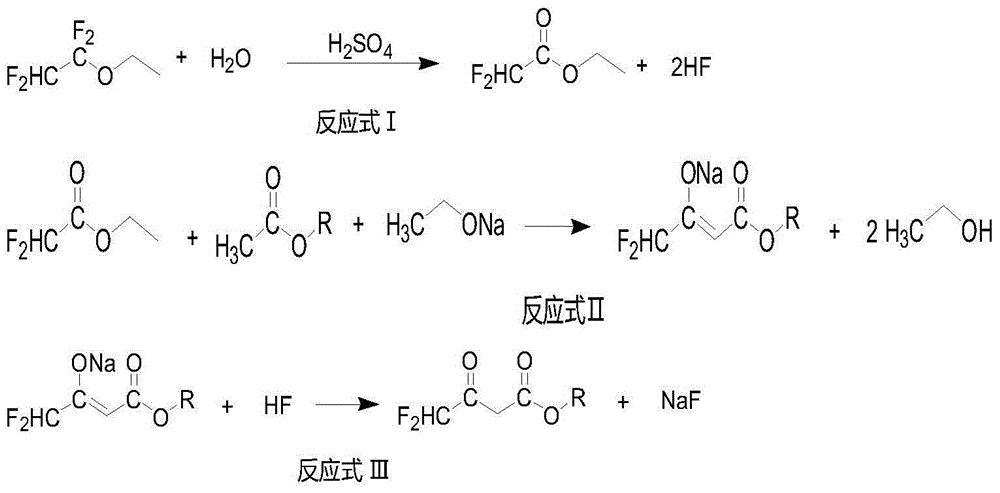
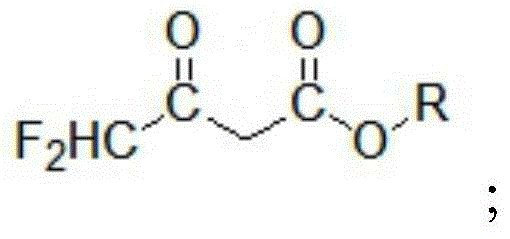
ActiveCN104447328BImprove responseImprove production efficiencyOrganic compound preparationCarboxylic acid esters preparationHydrogen fluoride2 Butenoic Acids
The invention discloses a method for preparing alkyl 4,4-difluoroacetylacetate. The method has the advantages of easily available raw materials, simple process flow, safety in operation and high yield. The method comprises the following steps: (1) carrying out hydrolysis reaction on 1,1,2,2-tetrafluoroethyl ether used as a raw material and water in an acid and separating to obtain ethyl difluoroacetate and hydrogen fluoride; (2) reacting ethyl difluoroacetate obtained in the step (1) and alkyl acetate in the presence of an alkaline catalyst to obtain 1,1-difluoro-2-butenoic acid alkyl ester-2-hydroxy salt; and (3) neutralizing 1,1-difluoro-2-butenoic acid alkyl ester-2-hydroxy salt obtained in the step (2) and hydrogen fluoride to obtain alkyl 4,4-difluoroacetylacetate.
Owner:KINGCHEM LIAONING CHEMICAL CO LTD
4-(N-phenthiazine-)-carbonyl-2-butenoic acid and preparation method thereof
InactiveCN103073523BSimple and fast operationReaction is easy to controlOrganic chemistry2 Butenoic AcidsFiltration
The invention discloses 4-(N-phenthiazine-)-carbonyl-2-butenoic acid and a preparation method thereof. A structural formula of 4-(N-phenthiazine-)-carbonyl-2-butenoic acid is as shown in the specification. The preparation method of 4-(N-phenthiazine-)-carbonyl-2-butenoic acid comprises the steps that A mol phenthiazine and B mol maleic anhydride are added to a reaction vessel; then C ml DMF (Dimethyl Formamide) is added; reaction liquid is obtained; the reaction liquid is heated to 100-155 DEG C on a condition of stirring and subjected to heat preservation; TLC (thin layer chromatography) is used for monitoring a reaction process; the reaction liquid is stirred continuously at 100-155 DEG C for 0.5h after raw material points are disappeared; a mixture is obtained, wherein the ratio of A to B to C is equal to 1:(1.0-1.3)-(400-800); after the mixture is cooled to a room temperature, the mixture is poured into water, subjected to suction filtration, washed and dried; and 4-(N-phenthiazine-)-carbonyl-2-butenoic acid is obtained. The method is easy and simple to operate; the reaction process is easy to control; post treatment is simple; and the productivity is high.
Owner:SHAANXI UNIV OF SCI & TECH
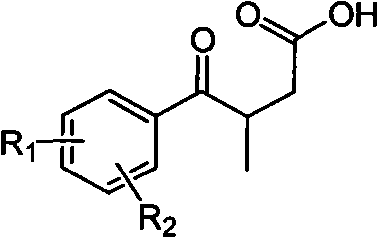
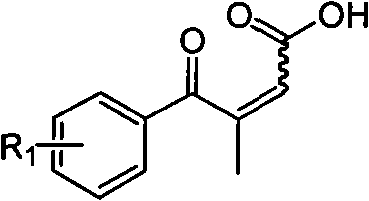
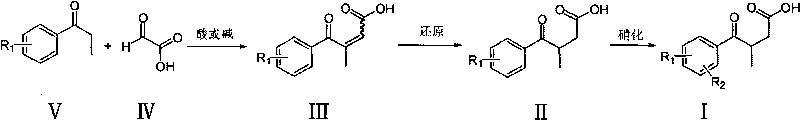
Novel process for preparing 3-(substituted benzoyl) butyric acid
InactiveCN101704740AOrganic compound preparationCarboxylic compound preparationAcetic acid2 Butenoic Acids
The invention provides a novel process, which takes substituted propiophenone and oxoacetic acid as starting raw materials, carries out condensation to obtain 3-(substituted benzoyl)-2-butenoic acid, and carries out reduction and nitrification to obtain a pimobendan intermediate 3-(substituted benzoyl) butyric acid. The method has the advantages of few steps, easy and simple operation, easy separation and purification of products, high yield and low cost, uses common reagents, and is the novel process suitable for industrialized production.
Owner:北京信益泰医药科技开发有限公司
Method for preparing alkyl 4,4-difluoroacetylacetate
ActiveCN104447328AImprove responseImprove production efficiencyOrganic compound preparationCarboxylic acid esters preparationHydrogen fluoride2 Butenoic Acids
The invention discloses a method for preparing alkyl 4,4-difluoroacetylacetate. The method has the advantages of easily available raw materials, simple process flow, safety in operation and high yield. The method comprises the following steps: (1) carrying out hydrolysis reaction on 1,1,2,2-tetrafluoroethyl ether used as a raw material and water in an acid and separating to obtain ethyl difluoroacetate and hydrogen fluoride; (2) reacting ethyl difluoroacetate obtained in the step (1) and alkyl acetate in the presence of an alkaline catalyst to obtain 1,1-difluoro-2-butenoic acid alkyl ester-2-hydroxy salt; and (3) neutralizing 1,1-difluoro-2-butenoic acid alkyl ester-2-hydroxy salt obtained in the step (2) and hydrogen fluoride to obtain alkyl 4,4-difluoroacetylacetate.
Owner:KINGCHEM LIAONING CHEMICAL CO LTD
Method for preparing 4-(N-phenothiazine)-carbonyl-2-butenoic acid
ActiveCN103232412AImprove catalytic performanceSimple and fast operationOrganic chemistry2 Butenoic AcidsDistillation
The invention relates to a method for preparing 4-(N-phenothiazine)-carbonyl-2-butenoic acid. The method comprises the following steps: adding A mol of phenothiazine and B mol of maleic anhydride into a reaction container, and then adding acetonitrile; dripping concentrated hydrochloric acid into the reaction container while stirring to get a reaction solution, increasing the temperature of the reaction solution from room temperature to 40-80 DEG C, and performing reaction for 1-2.5h while stirring to get a mixed solution, wherein A: B=2.5: (2.5-3.0); performing reduced-pressure distillation on the mixed solution to remove acetonitrile to get a concentrated solution, wherein the volume of acetonitrile in the concentrated solution is 15-20% of the volume of acetonitrile in the mixed solution; and cooling the concentrated solution to room temperature, then adding water into the concentrated solution, uniformly stirring, then performing suction filtration, washing a filter cake with water, and drying to get 4-(N-phenothiazine)-carbonyl-2-butenoic acid. According to the method disclosed by the invention, acetonitrile is adopted as a solvent, concentrated hydrochloric acid is taken as a catalyst, and the method has the advantages of simplicity and convenience in operation, short reaction time, mild conditions, simple post-treatment, low price and easiness in obtaining of raw materials and high yield.
Owner:SHAANXI UNIV OF SCI & TECH
A method for synthesizing 2-amido-4-(o-alkylphosphonyl)-2-butenoic acid and esters thereof
The invention belongs to the technical field of pesticide intermediates for synthesizing herbicides and particularly relates to a method for synthesizing a pesticide intermediate 2-amido-4-(O-alkylmethylphosphonyl)-2-butenoic acid and its ester. The method comprises that a compound VII and a compound VI undergo an Arbuzov reaction to produce a compound V, the compound V is hydrolyzed through an acid to produce a compound IV, the compound IV and a compound III undergo a reaction under action of a condensation reagent and a catalyst to produce a compound II and the compound II is hydrolyzed into a compound I. Compared with the existing synthesis route, the method has simple processes, high atom economy and high stereoselectivity, is environmentally friendly, realizes a low cost and has an industrial prospect. In the formula, R1 represents C1-4 alkyl; R2 and R3 represent CnH2n+1 and n is 1, 2, 3 or 4, or R2 or R3 represents -CmH2m- and m is 2 or 3; R4 represents C1-4 alkyl or C6-10 aryl; R5 represents hydrogen or C1-4 alkyl; and X represents chlorine or bromine.
Owner:NANJING TECH UNIV
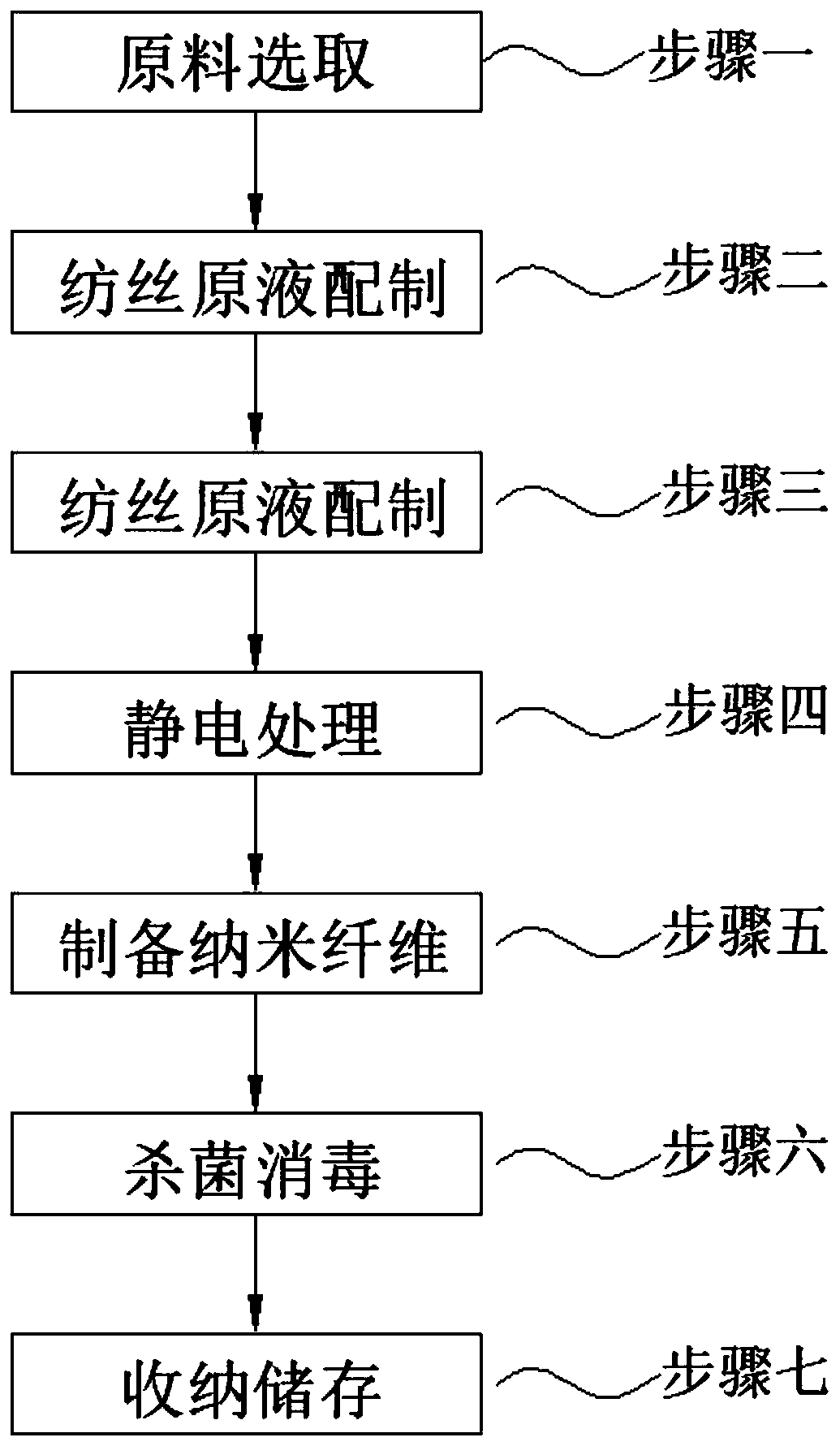
Preparation method of solvent-type nanofiber
InactiveCN110230114AEasy to useImprove finished product qualityArtificial thread manufacturing machinesElectro-spinningMethacrylateFiber
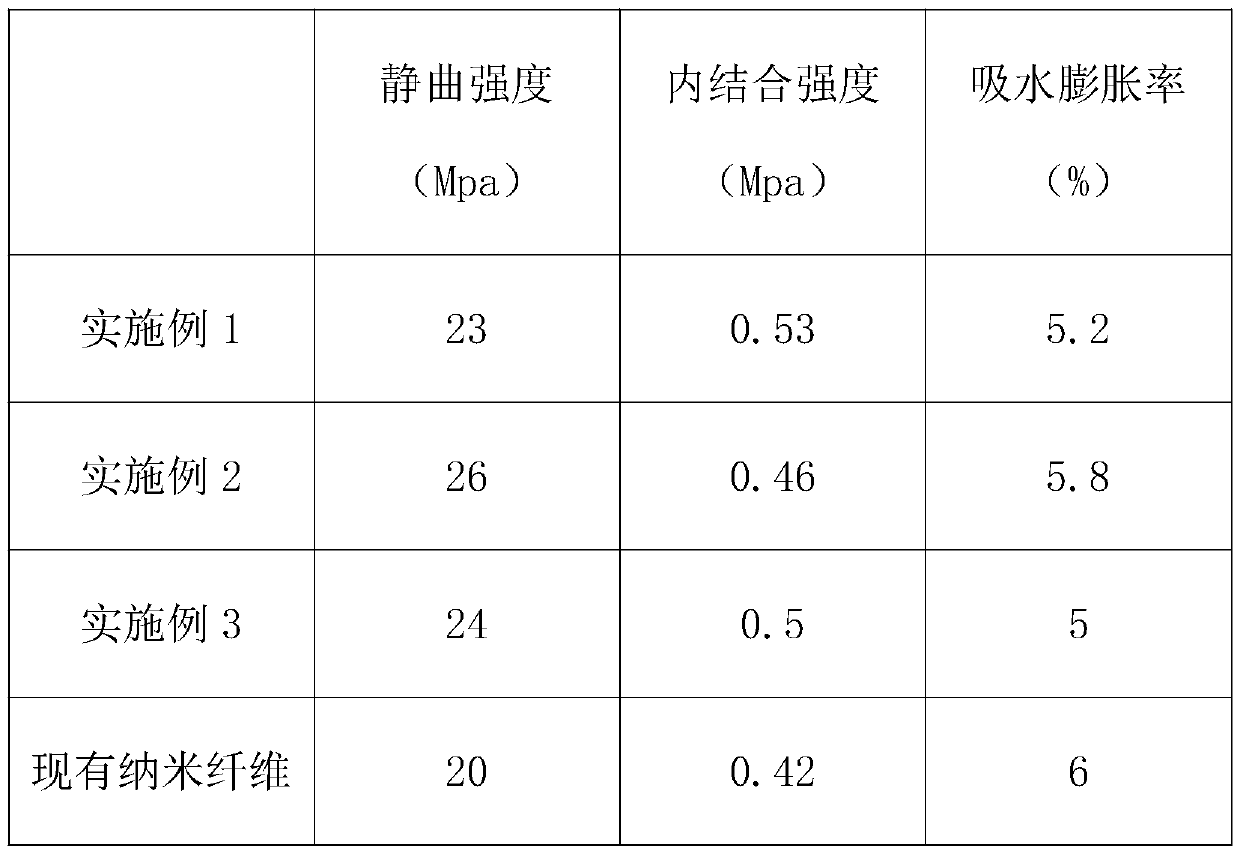
The invention discloses a preparation method of solvent-type nanofiber. The preparation method comprises the following steps: 1, selecting raw materials; 2, preparing a spinning stock solution; 3, performing ultrasonic vibration treatment; 4, performing electrostatic treatment; 5, preparing the nanofiber; 6, sterilizing and disinfecting; 7, storing, wherein in the step 1, in mass percentage, the raw materials include 15-26% of ethyl methacrylate, 12-15% of isobutyl acrylate, 1-5% of beta-butyl itaconate, 15-25% of 2-butenoic acid, 3-11% of methyl maleic acid, 8-11% of acrylonitrile and 25-45%of dimethyl sulfoxide, and the raw materials are weighed according to the sum 1 of weight percentages. By the preparation method, the steps are perfected and the temperature and equipment in the method are normalized, so that the nanofiber prepared in the later stage has very good static bending strength and internal bonding strength under the premise that good characteristics of the original nanofiber are maintained, and thus use of the nanofiber in the later stage is facilitated.
Owner:北京百年初心科技有限公司
Edaravone derivative and preparation and detection methods and application thereof
InactiveCN109134374AHigh purityEasy to prepareOrganic chemistryComponent separation2 Butenoic AcidsOrganic solvent
The invention relates to a compound which is shown in the following structure and preparation and detection methods and application of the compound. The preparation method is characterized by comprising the steps that edaravone is dissolved in an organic solvent and then reacts with an aqueous hydrogen peroxide solution, the reversed-phase high-performance liquid chromatography is used for separating an edaravone reaction liquid, and (Z)-2-(3-methy-5-oxygen-1-phenyl-4,5-dihydro-1H-pyrazol-4-radical)-3-[(E)-phenylazo]-2-butenoic acid is prepared. In addition, the invention further provides thedetection method of the compound. The compound can be applied to an edaravone impurity reference substance, and the amount of edaravone raw material medicine and the amount of the compound of the related preparation are conveniently controlled. The compound is shown in the description.
Owner:JIANGSU SIMCERE PHARMA +1
Method for preparing palbociclib
InactiveUS20170247379A1Improve availabilitySimple and direct processOrganic active ingredientsAmino group formation/introductionDehydrogenationMethyl group
The invention discloses a method for preparing Palbociclib (I). The preparation method comprises the steps of: causing a ring-closing reaction of 2-acetyl-2-butenoic acid methyl ester and malononitrile to occur in an alkaline condition to generate 1,4,5,6-tetrahydro-2-methoxyl-4-methyl-5-acetyl-6-oxy-3-pyridine carbonitrile (II); causing a substitution reaction of the intermediate(II) and halogenated cyclopentane(III) to occur under the effect of acid binding agent to generate N-cyclopentyl-1,4,5,6-tetrahydro-2-methoxyl-4-methyl-5-acetyl-6-oxy-3-pyridinecarbonitrile (IV); causing a condensation reaction of the intermediate(IV) and N-[5-(1-piperazinyl)-2-pyridinyl]guanidine (V) to occur to generate 6-acetyl-8-cyclopentyl-5,8-dihydro-5-methyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-pyrido[2,3-d]pyrimidin-7(6H)-one (VI); and causing a dehydrogenation reaction of the intermediate(VI) and sodium selenate to occur to prepare Palbociclib(I).The preparation method has readily available raw materials and a simple process, is economical and environmentally friendly, and is suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH
Synthesis method of pharmaceutical intermediate for treating arrhythmia
The invention belongs to the technical field of synthesis of pharmaceutical intermediates, and in particular relates to a synthesis method of a pharmaceutical intermediate for treating arrhythmia. Themethod adopts ammonia gas, acrylate and 2-butenoic acid as raw materials, and the target product is obtained by six steps; in the second step, a primary amine compound 2 is obtained by enabling the ammonia gas to react with butenolide; in the third step, the acrylate and the compound 2 are enabled to react so as to obtain a compound 3; finally, the target product is synthesized by using a diestersynthesis method. The yield of a whole route reaches up to 40% or above.
Owner:JINAN SHUNJING PHARMA TECH
Preparation of (E)- and (Z)-2-methyl-2-butenoic acids
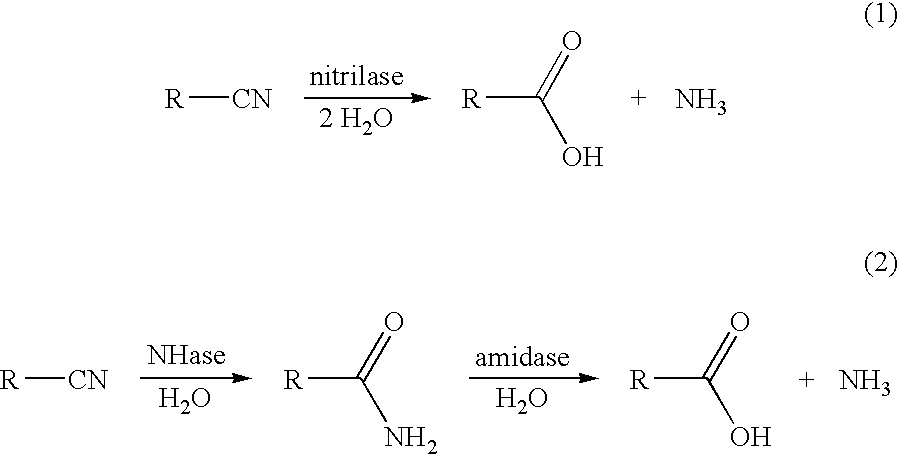
InactiveUS20060115883A1High regional selectivityHigh purityBacteriaHydrolases2 Butenoic AcidsRegioselectivity
A method has been developed to prepare (E)- and (Z)-2-methyl-2-butenoic acids (2M2BA) from a mixture of (E,Z)-2-methyl-2-butenenitriles (2M2BN) by the regioselective hydrolysis of (E)-2M2BN to (E)-2-methyl-2-butenoic acid (2M2BA) using enzyme catalysts having either a nitrilase activity or a combination of nitrile hydratase and amidase activities. The method provides high yields without significant conversion of (Z)-2M2BN to (Z)-2M2BA. The regioselective hydrolysis of (E)-2M2BN to (E)-2M2BA makes possible the facile separation of (E)-2M2BA from (Z)-2M2BN or (Z)-2-methyl-2-butenamide (2M2BAm), and the subsequent conversion of (Z)-2M2BN or (Z)-2M2BAm to (Z)-2M2BA.
Owner:EI DU PONT DE NEMOURS & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com