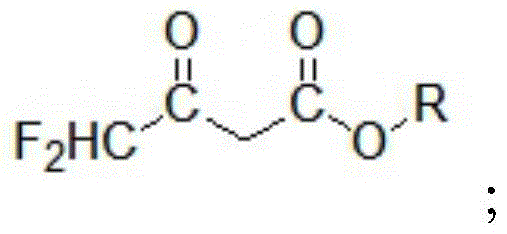
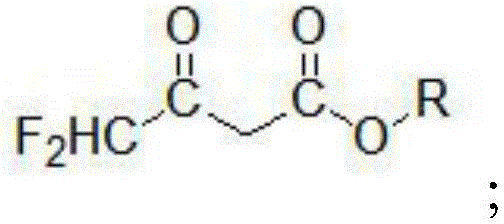
A kind of preparation method of 4,4-difluoroacetoacetate alkyl ester
A technology of alkyl difluoroacetoacetate and alkyl acetate, which is applied in the field of preparation of alkyl 4,4-difluoroacetoacetate, can solve the problems such as the complicated preparation process of intermediate ethyl difluoroacetate, and achieve Simplify the reaction process, improve the safety factor, and benefit the effect of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
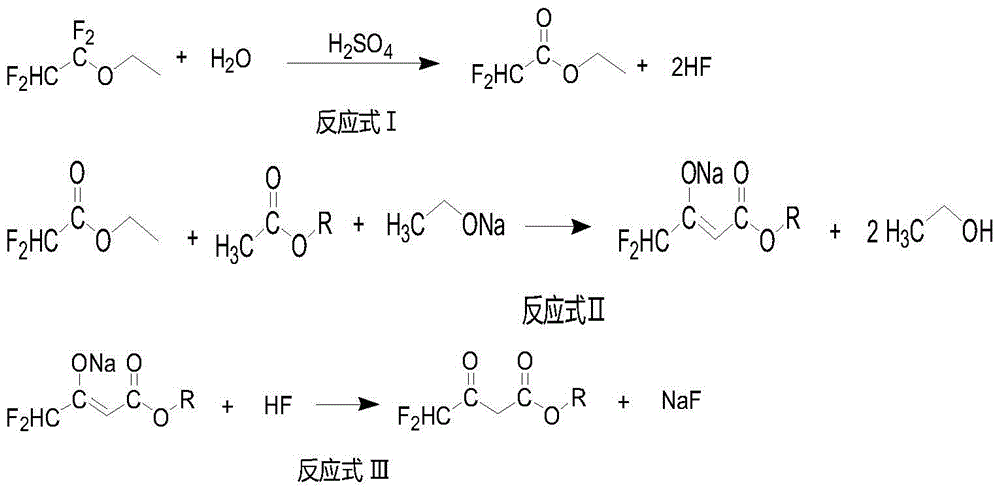
[0039] In a 1000mL Teflon-lined reactor, add 243g (2.4mol) of 98% sulfuric acid, cool down in a water bath, control the temperature at 10-30°C, slowly add 57g (3.2mol) of water dropwise, and then slowly add 1,1, 438 g (3.0 mol) of 2,2-tetrafluoroethyl ethyl ether. After the dropwise addition, the temperature was raised to 54-56° C. for 1 hour. At this time, there is almost no gas overflow, and the temperature is slowly raised to 80° C., and hydrogen fluoride gas overflows (pass the overflow gas into the next reaction). Lower the temperature of the system to 10-20°C, control the temperature T<30°C, and separate the hydrogen fluoride by rectifying the system under reduced pressure (separate the hydrogen fluoride and pass it into the next step reaction). The crude ethyl difluoroacetate was evaporated, washed with water, washed with sodium bicarbonate solution, washed with water and rectified to obtain 316.0 g of ethyl difluoroacetate with a yield of 85%.
[0040] In a 1000mL gl...
Embodiment 2
[0042] In a 1000ml polytetrafluoroethylene-lined reactor, add 243g (2.4mol) of 98% sulfuric acid, cool down in a water bath, control the temperature at 10-30°C, slowly add 57g (3.2mol) of water dropwise, and then slowly add 1,1,2 , 438 g (3.0 mol) of 2-tetrafluoroethyl ethyl ether. After the dropwise addition, the temperature was raised to 54-56° C. for 1 hour. At this time, there is almost no gas overflow, and the temperature is slowly raised to 80° C., and hydrogen fluoride gas overflows (pass the overflow gas into the next reaction). Lower the temperature of the system to 10-20°C, control the temperature T<30°C, and separate the hydrogen fluoride by rectifying the system under reduced pressure (separate the hydrogen fluoride and pass it into the next step reaction). Evaporate the crude product of ethyl difluoroacetate, control the temperature of the crude product at T<10°C, pass through ammonia gas, adjust the pH=7, filter, rinse the filter cake with 200g of ethyl acetate,...
Embodiment 3
[0045] In a 1000ml polytetrafluoroethylene-lined reactor, add 243g (2.4mol) of 98% sulfuric acid, cool down in a water bath, control the temperature at 10-30°C, slowly add 57g (3.2mol) of water dropwise, and then slowly add 1,1,2 , 438 g (3.0 mol) of 2-tetrafluoroethyl ethyl ether. After the dropwise addition, the temperature was raised to 54-56° C. for 1 hour. At this time, there is almost no gas overflow, and the temperature is slowly raised to 80° C., and hydrogen fluoride gas overflows (pass the overflow gas into the next reaction). Lower the temperature of the system to 10-20°C, control the temperature T<30°C, and separate the hydrogen fluoride by rectifying the system under reduced pressure (separate the hydrogen fluoride and pass it into the next step reaction). Evaporate the crude product of ethyl difluoroacetate, control the temperature of the crude product at T<10°C, pass through ammonia gas, adjust the pH=7, filter, rinse the filter cake with 200g of ethyl acetate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com