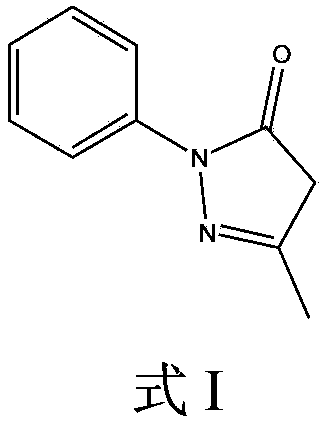
Edaravone derivative, and preparation method, detection method and application thereof
A compound and aqueous solution technology, applied in the field of edaravone derivatives, achieves the effects of simple preparation method, high product purity and simple detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of compound A
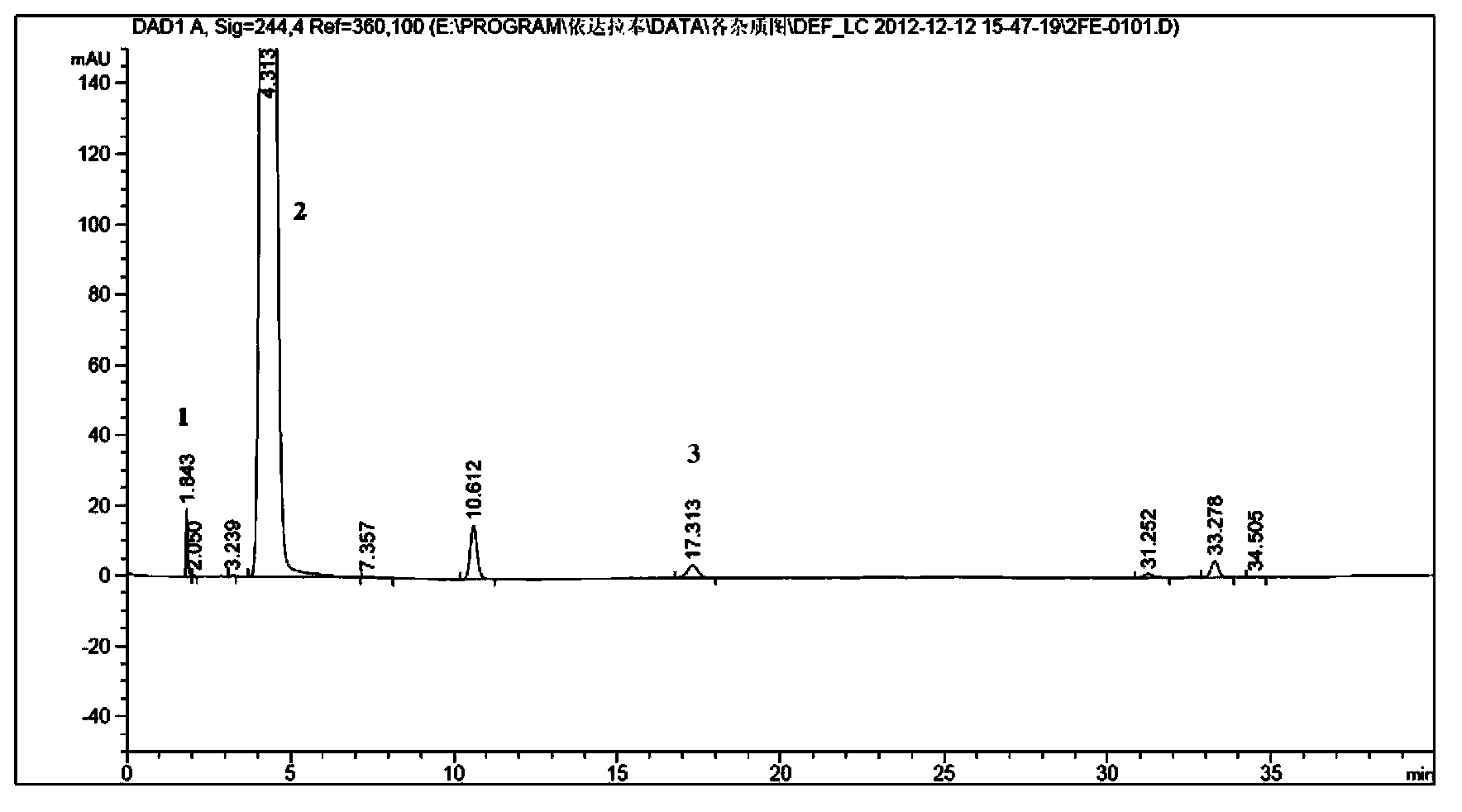
[0029] Take 10g of Edaravone raw material drug and add methanol 200ml to dissolve, slowly add 50ml of hydrogen peroxide with a mass fraction of 30%, stir at room temperature 20°C for 48 hours, spin off part of the solvent at 10°C, and prepare the solution for later use.
[0030] Then adopt reverse-phase high performance liquid chromatography to separate above-mentioned solution, chromatographic condition is: chromatographic column is Waters Nova-Pak C18, 6 μ m, 190*300mm, mobile phase a is methyl alcohol: water: glacial acetic acid=45:55:0.1 , mobile phase b is methanol, flow rate: 20ml / min, column temperature: 20°C, detection wavelength: 244nm, injection volume: 0.5ml. Gradient elution is as follows:
[0031] time (min)
Proportion of mobile phase a (%)
Proportion of mobile phase b (%)
0
100
0
22
100
0
52
36
64
72
36
64
80
100
0
...
Embodiment 2
[0044] Embodiment 2: the preparation of compound A
[0045] Take 10g of Edaravone raw material drug and add 200ml of acetonitrile to dissolve, slowly add 50ml of hydrogen peroxide with a mass fraction of 30%, stir at room temperature 20°C for 48 hours, spin off part of the solvent at 10°C, and prepare the solution for later use.
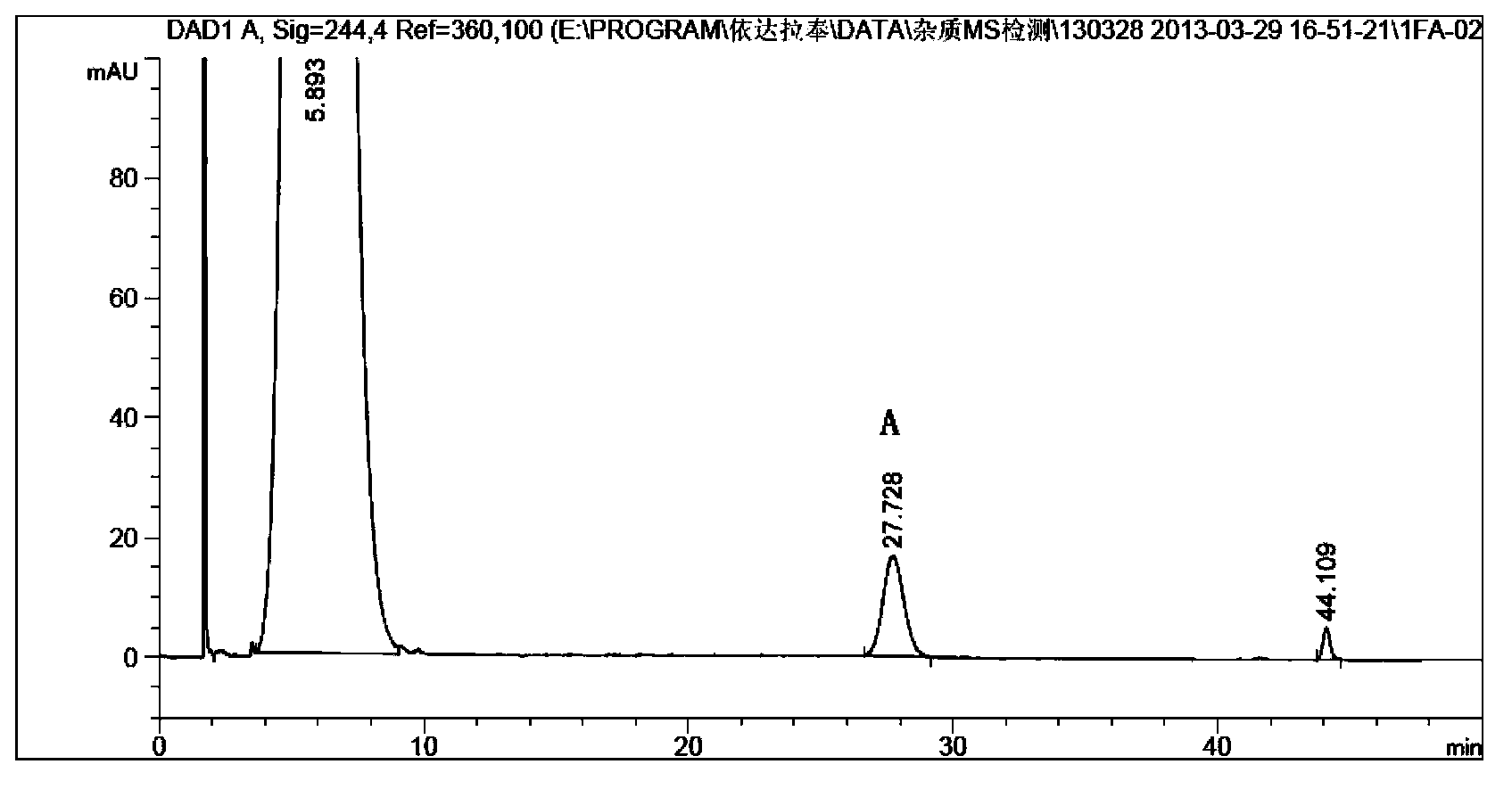
[0046] Then adopt reverse-phase high performance liquid chromatography to separate above-mentioned solution, chromatographic condition is: chromatographic column is Waters Nova-Pak C18, 6 μ m, 190*300mm, mobile phase a is water: glacial acetic acid=99.9:0.1, mobile phase b Acetonitrile, flow rate: 20ml / min, column temperature: 20°C, detection wavelength: 244nm, injection volume: 0.5ml. Gradient elution is as follows:
[0047] time (min)
[0048] The preparation solution with a relative retention time of 2.3-2.9 was collected, and the solvent was spin-dried at 10°C to obtain 5 mg of compound A with a yield of 0.07%.
Embodiment 3
[0049] Embodiment 3: the detection of compound A
[0050] Preparation of sample: Accurately weigh 10 mg of Edaravone crude drug, place it in a 50ml volumetric flask, dissolve and settle to volume with methanol: water: glacial acetic acid=45:55:0.1 mixed solution, configure Edaravone content The sample is 0.2mg / ml, the temperature is controlled at 4°C, and it is prepared and prepared within half an hour.
[0051] Chromatographic conditions and detection:
[0052] Instrument: Agilent1200HPLC
[0053] Chromatographic column: Agilent Eclipse XDB-C18, 4.6*150mm (3.5μm);
[0054]Mobile phase a is methanol:water:glacial acetic acid=45:55:0.1, mobile phase b is methanol, flow rate: 0.8ml / min, column temperature: 20°C, detection wavelength: 244nm, injection volume: 20ml. Gradient elution is as follows:
[0055] time (min)
[0056] Test results: The peak eluting time of compound A was 17.3 min, and the peak eluting time of raw material phenylhydrazine was 1.8 min. (attach...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com