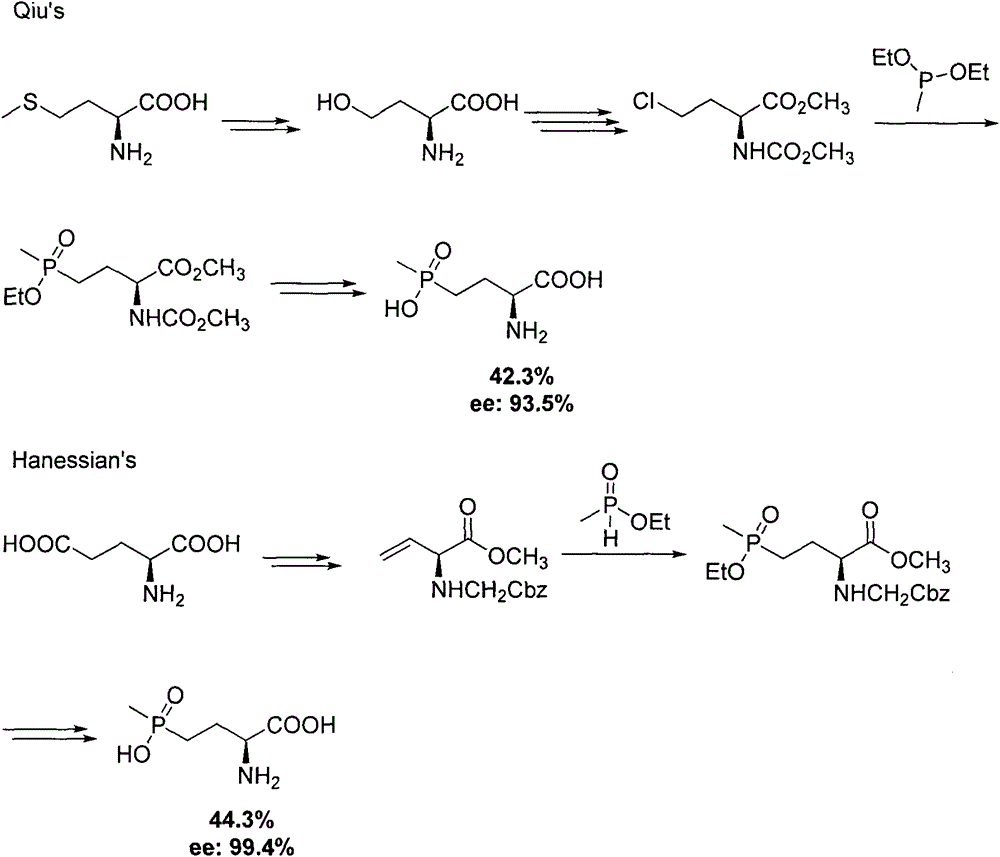
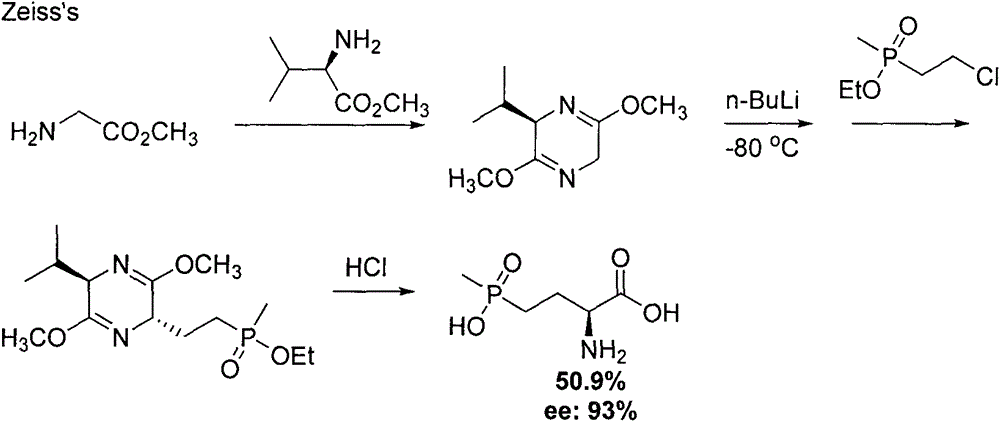
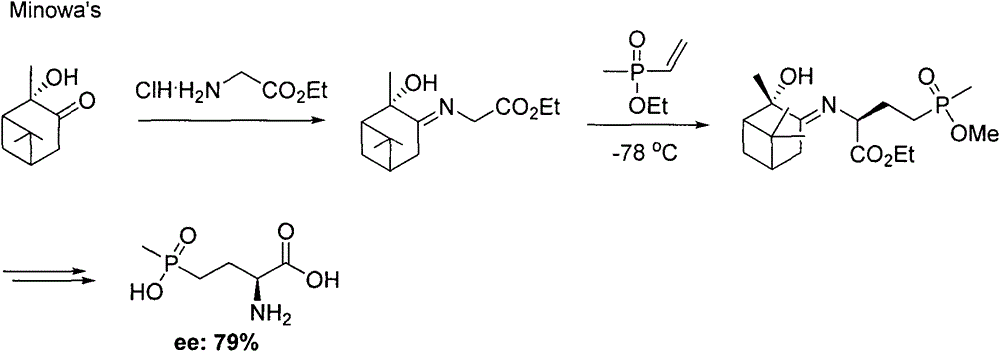
Method for synthesizing pesticide intermediate 2-amido-4-(O-alkylmethylphosphonyl)-2-butenoic acid and its ester
A technology of alkylmethylphosphono, synthesis method, applied in the field of chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of 2-(O-methylmethylphosphono)acetaldehyde diethyl acetal (compound V)
[0044] A 250 mL round bottom flask was charged with diethyl methyl phosphonite (compound VII) (54.0 g, 0.5 mol) and bromoacetaldehyde diethyl acetal (98.5 g, 0.5 mol). Heating, refluxing reaction at 120°C for 2h (GC monitoring reaction), cooling to room temperature, distilling the reaction solution under reduced pressure, collecting 120°C fraction (Compound V) 84.2g under 15mmHg pressure, yield 80.2%.
Embodiment 2
[0045] Example 2: Preparation of 2-(O-ethylmethylphosphono)acetaldehyde diethyl acetal (compound V)
[0046] A 500 mL round bottom flask was charged with diethyl methylphosphonite (compound VII) (68.0 g, 0.5 mol), bromoacetaldehyde diethyl acetal (98.5 g, 0.5 mol) and toluene (200 ml). Heating, refluxing reaction at 110°C for 4h (GC monitoring reaction), cooling to room temperature, distilling the reaction solution under reduced pressure, collecting 120°C fraction (compound V) 101.9g under 15mmHg pressure, yield 91.0%, 1 H NMR(400MHz, CDCl 3 ,Δppm): 1.22(td, J 1 =6.8Hz, J 2 = 3.2 Hz, 6H), 1.33 (t, J = 6.8 Hz, 3H), 1.53 (d, J = 14.4 Hz, 3H), 2.16 (pd, J 1 =15.8Hz, J 2 =5.2Hz, 2H), 3.62 (m, 4H), 4.06 (m, 2H), 4.88 (dd, J 1 =11.4Hz, J 2 =5.2Hz, 1H), 31 P NMR(162MHz, CDCl 3 , Δppm): 51.06.
Embodiment 3
[0047] Example 3: Preparation of 2-(O-isopropylmethylphosphono)acetaldehyde diethyl acetal (compound V)
[0048] Add O, O-diisopropyl methylphosphonite (Compound VII) (82.0g, 0.5mol), bromoacetaldehyde diethyl acetal (98.5g, 0.5mol) and acetonitrile into a 500mL round bottom flask (200ml). Heating, refluxing reaction at 80°C for 24h (GC monitoring reaction), cooling to room temperature, distilling the reaction solution under reduced pressure, collecting 122°C fraction (compound V) 86.0g under 15mmHg pressure, yield 72.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com