Edaravone derivative and preparation and detection methods and application thereof
A compound and aqueous solution technology, applied in the field of edaravone derivatives, achieves the effects of simple preparation method, high product purity, and simple detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
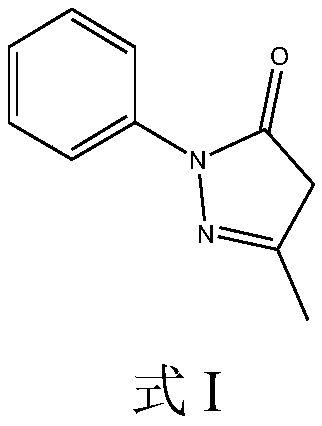
[0029] Embodiment 1: the preparation of compound A
[0030] Take 10g of edaravone raw material drug and add methanol 200ml to dissolve, slowly add 50ml of hydrogen peroxide with a mass fraction of 30%, stir at room temperature 20°C for 48 hours, spin off part of the solvent at 10°C, and prepare the solution for later use.
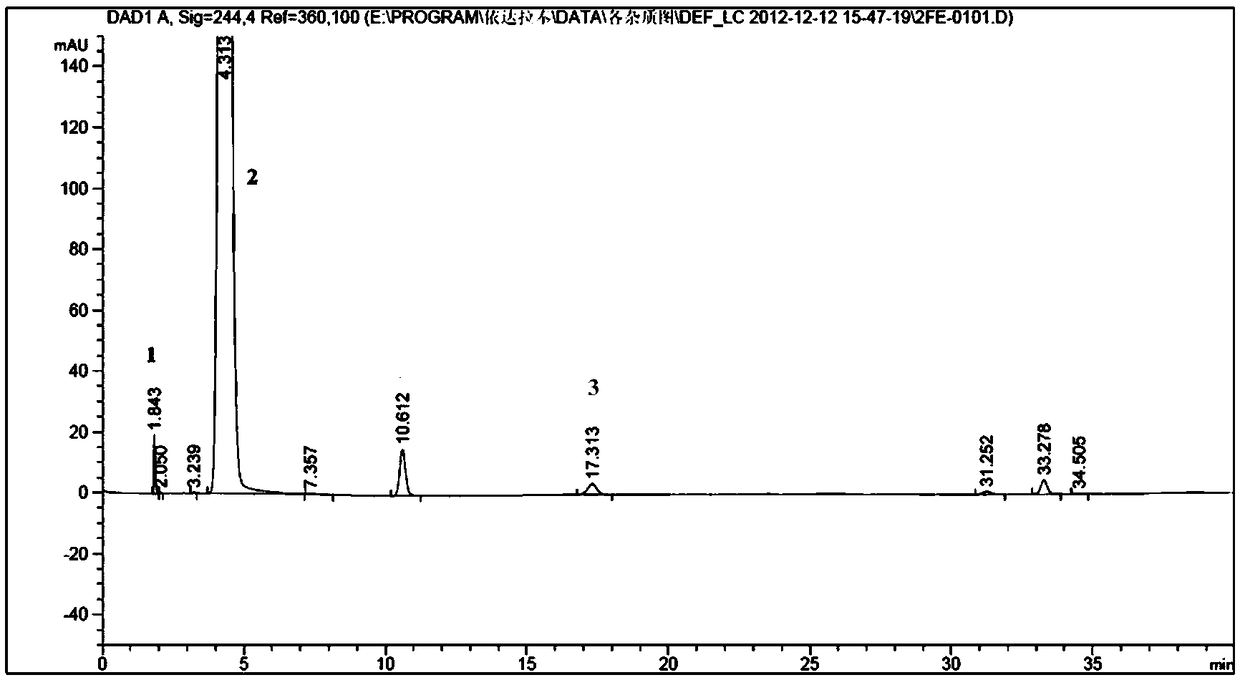
[0031] Then adopt reverse-phase high-performance liquid chromatography to separate above-mentioned solution, chromatographic condition is: chromatographic column is Waters Nova-Pak C18, 6 μm, 190*300mm, mobile phase a is methanol: water: glacial acetic acid=45:55:0.1 , mobile phase b is methanol, flow rate: 20ml / min, column temperature: 20°C, detection wavelength: 244nm, injection volume: 0.5ml. Gradient elution is as follows:
[0032] time (min)
Proportion of mobile phase a (%)
Proportion of mobile phase b (%)
0
100
0
22
100
0
52
36
64
72
36
64
80
100
0
[0033] The prepara...
Embodiment 2
[0045] Embodiment 2: the preparation of compound A
[0046] Take 10 g of Edaravone raw material drug and add 200 ml of acetonitrile to dissolve, slowly add 50 ml of hydrogen peroxide with a mass fraction of 30%, stir at room temperature 20°C for 48 hours, spin off part of the solvent at 10°C, and prepare the solution for later use.
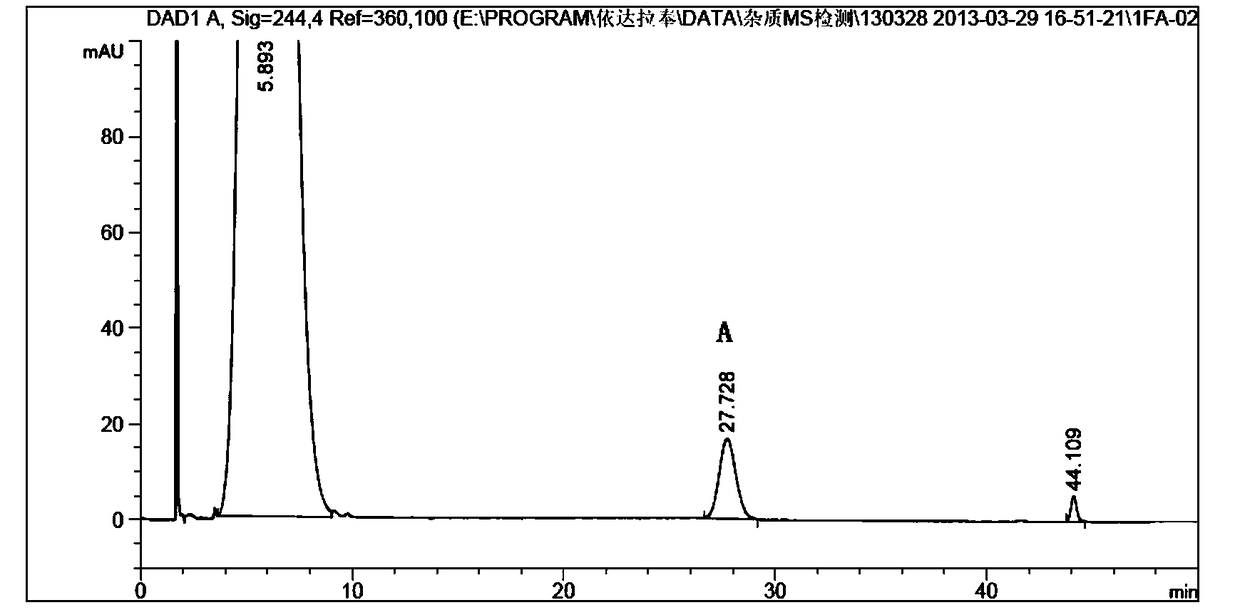
[0047] Then adopt reverse-phase high performance liquid chromatography to separate above-mentioned solution, chromatographic condition is: chromatographic column is Waters Nova-Pak C18, 6 μm, 190*300mm, mobile phase a is water: glacial acetic acid=99.9:0.1, mobile phase b Acetonitrile, flow rate: 20ml / min, column temperature: 20°C, detection wavelength: 244nm, injection volume: 0.5ml. Gradient elution is as follows:
[0048] time (min)
[0049] The preparation solution with a relative retention time of 2.3-2.9 was collected, and the solvent was spin-dried at 10° C. to obtain 5 mg of compound A with a yield of 0.07%.
Embodiment 3
[0050] Embodiment 3: the detection of compound A
[0051] The preparation of sample: precision takes by weighing edaravone crude drug 10mg, is placed in 50ml volumetric flask, dissolves and constant volume with methanol: water: the mixed solution of glacial acetic acid=45:55:0.1, is configured to the content of edaravone The sample is 0.2mg / ml, the temperature is controlled at 4°C, and it is prepared and prepared within half an hour.
[0052] Chromatographic conditions and detection:
[0053] Instrument: Agilent 1200 HPLC
[0054] Chromatographic column: Agilent Eclipse XDB-C18, 4.6*150mm (3.5μm);
[0055] Mobile phase a is methanol:water:glacial acetic acid=45:55:0.1, mobile phase b is methanol, flow rate: 0.8ml / min, column temperature: 20°C, detection wavelength: 244nm, injection volume: 20ml. Gradient elution is as follows:
[0056] time (min)
[0057] Test results: The peak eluting time of compound A was 17.3 min, and the peak eluting time of raw material pheny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com