Synthesis method of pharmaceutical intermediate for treating arrhythmia
A synthetic method and arrhythmia technology, applied in the direction of organic chemistry, etc., can solve the problems of low yield of asymmetric diester and low yield of piperidone products, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
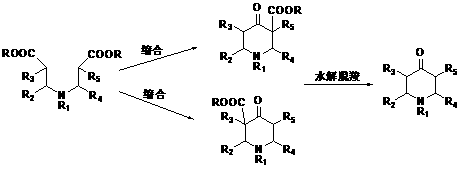
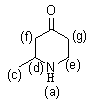
[0036] The technical solution provided by the examples in this embodiment is: a method for synthesizing a drug intermediate for treating arrhythmia, wherein the drug intermediate for treating arrhythmia is 2-methyl-4-piperidone; The synthetic method comprises the steps:
[0037] Step 1: Trans-2-butenoic acid and ethanol undergo esterification reaction to obtain compound 1;
[0038] Step 2: Compound 1 is obtained through addition reaction with ammonia gas to obtain Compound 2;
[0039] Step 3: compound 2 and ethyl acrylate are added to obtain compound 3;
[0040] Step 4: react compound 3 with ethyl chloroformate to protect the amine group to obtain compound 4;
[0041] Step 5: compound 4 is ring-closed to obtain compound 5 through Dickmann condensation reaction;
[0042] Step 6: compound 5 is hydrolyzed to obtain compound 6;
[0043] in:
[0044] .
Embodiment 1
[0047] The technical scheme of the synthetic method of the pharmaceutical intermediate 2-methyl-4-piperidone provided in this example is that the specific operations of step 1 include: 25.03 g (0.29 mol) trans-2-butenoic acid, 64 mL Add absolute ethanol and 2.50 g of concentrated sulfuric acid into a 250 mL three-necked flask equipped with a thermometer, reflux condenser and drying tube, stir magnetically, heat and reflux for 5 h, pour the reaction solution into 250 ml of water, and after thorough stirring, use two Extracted three times with methyl chloride, combined the organic phases, washed with water until neutral, dried over anhydrous sodium sulfate, distilled the obtained crude product under atmospheric pressure, collected fractions at 138-140°C, and obtained compound 1 as a colorless liquid with a yield of 96.6%
[0048] The specific operation of step 2 includes: adding 60 mL of absolute absolute ethanol to a dry 100 mL single-necked bottle, and feeding 0.33 mol of ammon...
Embodiment 2-3 and comparative example 1-2
[0059] The difference between embodiment 2-3 and comparative example 1-2 and embodiment 1 is that the reaction temperature and time of step 2 and step 3 are different. Concrete temperature, time, productive rate are shown in Table 2.
[0060] Table 2 step two, three reaction conditions comparison
[0061]
[0062] It can be seen from the above table that the embodiment provided by the present invention is passed, and the reaction conditions of step 2 are controlled at: heating to reflux, reacting for 5-10 h, and at the same time controlling the reaction conditions of step 3 at 10-20°C for 18-36 h , the temperature was raised to 30-50°C for 2-5 h, which greatly improved the yield of a single step, thereby increasing the total yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com