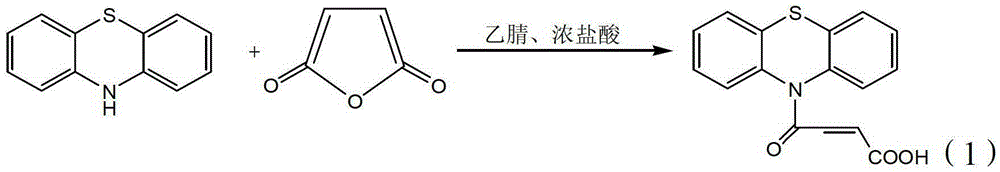
A method for preparing 4-(n-phenothiazine-)-carbonyl-2-butenoic acid
A technology of phenothiazine and crotonic acid, applied in the direction of organic chemistry, can solve the problems of long time, troublesome post-processing, high reaction temperature, etc., and achieve the effect of short reaction time, low equipment requirements and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the first step, add 0.0025mol of phenothiazine and 0.0025mol of maleic anhydride to a dry three-necked flask, then add 10mL of acetonitrile, and slowly add 0.2mL of mass concentration to the reaction vessel at a stirring speed of 1500r / min. 36-38% concentrated hydrochloric acid to obtain a reaction solution. The reaction solution was heated from room temperature at a rate of 3 °C / min to 40 °C, and stirred at a rate of 1500 r / min for 2.5 hours to obtain a mixed solution;
[0023] In the second step, the acetonitrile is evaporated from the mixed solution under reduced pressure to obtain a concentrated solution. The volume of acetonitrile in the concentrated solution is 15% of the volume of acetonitrile in the mixed solution. After the concentrated solution is cooled to room temperature, 10 mL of water is added thereto, stirred for 5 minutes, and pumped. Filter, wash the filter cake with water, and dry at room temperature to obtain a white powder that is 4-(N-phenothiazi...
Embodiment 2
[0026] In the first step, add 0.0025mol of phenothiazine and 0.0028mol of maleic anhydride to a dry three-necked flask, then add 10mL of acetonitrile, and slowly add 0.15mL of mass concentration to the reaction vessel at a stirring speed of 2200r / min. 36-38% concentrated hydrochloric acid to obtain a reaction solution, the reaction solution was heated from room temperature at a rate of 4 °C / min to 50 °C, and stirred at a rate of 2200 r / min for 2.5 hours to obtain a mixed solution;
[0027] In the second step, the acetonitrile is evaporated from the mixed solution under reduced pressure to obtain a concentrated solution. The volume of acetonitrile in the concentrated solution is 20% of the volume of acetonitrile in the mixed solution. After the concentrated solution is cooled to room temperature, 10 mL of water is added thereto, stirred for 5 minutes, and pumped. Filter, wash the filter cake with water, and dry at room temperature to obtain a white powder that is 4-(N-phenothiaz...
Embodiment 3
[0029] In the first step, add 0.00250mol of phenothiazine and 0.00275mol of maleic anhydride to a dry three-necked flask, then add 12mL of acetonitrile, and slowly add 0.2mL of mass concentration to the reaction vessel at a stirring speed of 1700r / min. 36-38% concentrated hydrochloric acid to obtain a reaction liquid, the reaction liquid was heated from room temperature at a rate of 3 °C / min to 60 °C, and stirred at a speed of 1700 r / min for 2 hours to obtain a mixed liquid;
[0030] In the second step, the acetonitrile is evaporated from the mixed solution under reduced pressure to obtain a concentrated solution. The volume of acetonitrile in the concentrated solution is 17% of the volume of acetonitrile in the mixed solution. After the concentrated solution is cooled to room temperature, 15 mL of water is added thereto, stirred for 7 minutes, and then pumped. Filter, wash the filter cake with water, and dry at room temperature to obtain a white powder that is 4-(N-phenothiazi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com