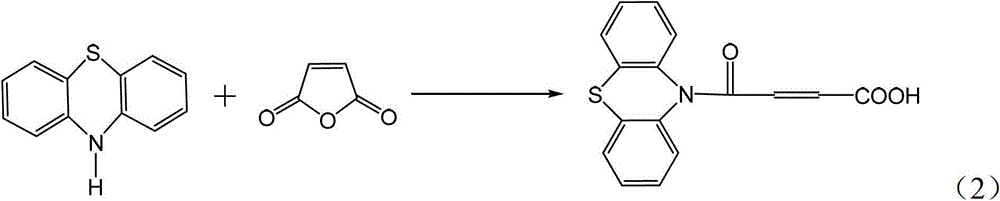
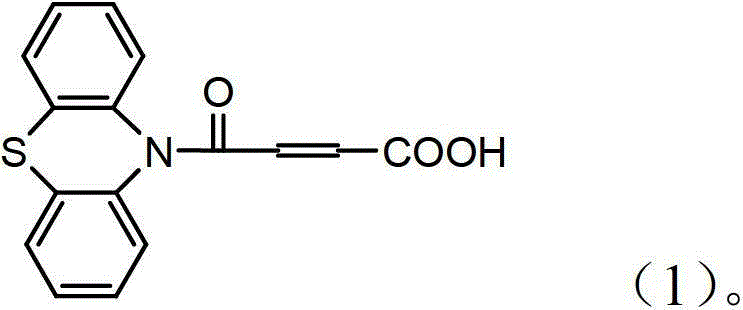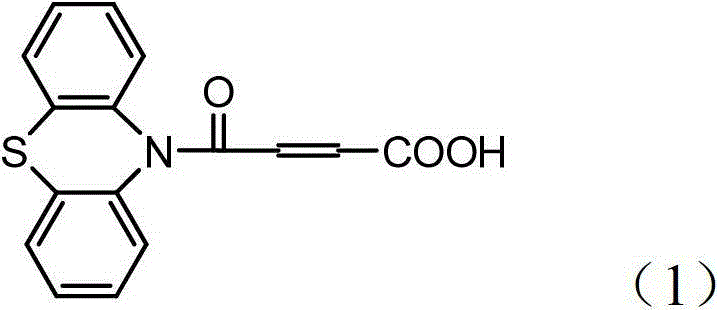4-(N-phenthiazine-)-carbonyl-2-butenoic acid and preparation method thereof
A technology of phenothiazine and crotonic acid, which is applied in the direction of organic chemistry, can solve the problems of no literature reports, etc., and achieve the effect of easy reaction process, high yield and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of 4-(N-phenothiazine-)-carbonyl-2-butenoic acid comprises the following steps:
[0025] In the first step, add 0.010mol of phenothiazine and 0.013mol of maleic anhydride to the dry reaction flask, and then add 8mL of DMF to obtain a reaction solution; at a stirring speed of 2000r / min, heat the reaction solution to 120°C And keep warm, use TLC to monitor the reaction process, after the raw material point disappears, continue to stir at 120 ° C for 0.5 h to obtain the mixture; wherein the developer of TLC is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:7;
[0026] In the second step, after the mixture is cooled to room temperature, it is poured into a beaker filled with 1.2 mol of water under stirring, suction filtered, washed with water, and dried at room temperature to obtain 2.77 g of white solid which is 4-(N-phenothiazine -)-oxo-2-butenoic acid, the yield was 93.27%. 1 H-NMR (CDCl 3 , 400M, TMS internal stand...
Embodiment 2
[0028] The preparation method of 4-(N-phenothiazine-)-carbonyl-2-butenoic acid comprises the following steps:
[0029] In the first step, add 0.020mol of phenothiazine and 0.021mol of maleic anhydride to the dry reaction flask, and then add 9mL of DMF to obtain a reaction solution; at a stirring speed of 1500r / min, heat the reaction solution to 130°C And keep warm, use TLC to monitor the reaction process, after the raw material point disappears, continue to stir at 130 ° C for 0.5 h to obtain the mixture; wherein the developer of TLC is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:6;
[0030] In the second step, after the mixture is cooled to room temperature, it is poured into a beaker containing 2.0 mol of water under stirring, suction filtered, washed with water, and dried at room temperature to obtain 5.56 g of white solid which is 4-(N-phenothiazine -)-oxo-2-butenoic acid, the yield was 93.60%.
Embodiment 3
[0032] The preparation method of 4-(N-phenothiazine-)-carbonyl-2-butenoic acid comprises the following steps:
[0033] In the first step, add 0.020mol of phenothiazine and 0.022mol of maleic anhydride to the dry reaction flask, and then add 10mL of DMF to obtain a reaction solution; at a stirring speed of 2500r / min, heat the reaction solution to 155°C And keep warm, use TLC to monitor the reaction process, after the raw material point disappears, continue to stir at 155 ° C for 0.5 h to obtain the mixture; wherein the TLC developer is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:8;
[0034] In the second step, after the mixture is cooled to room temperature, it is poured into a beaker containing 1.9 mol of water under stirring, suction filtered, washed with water, and dried at room temperature to obtain 5.55 g of white solid which is 4-(N-phenothiazine -)-oxo-2-butenoic acid, the yield was 93.43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com