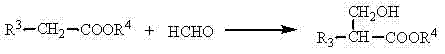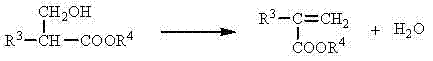Method of efficiently preparing alpha,beta-unsaturated carboxylic acids or esters
An unsaturated, carboxylic acid technology, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation and other directions, can solve the difficulty and cost of increasing separation, reduce the yield of the main product methyl methacrylate, etc. problem, to achieve the effect of low price, high yield and wide source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0029] 20 g γ-Al 2 o 3 Add 3.4 g KOH / 100 mL deionized water solution, soak for 6 h, calcined at 600 °C for 3 h, mechanically pulverize, and sieve out 20-30 mesh particles.
preparation example 2
[0031] 20 g γ-Al 2 o 3 Add 3.4 g KOH / 100 mL deionized water solution, soak for 6 h, calcined at 350 °C for 3 h, mechanically pulverize, and sieve out 20-30 mesh particles.
preparation example 3
[0033] 20 g γ-Al 2 o 3 Add 3.4 g KOH / 100 mL deionized water solution, soak for 6 h, calcined at 1200 °C for 3 h, mechanically pulverize, and sieve out 20-30 mesh particles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com