Preparation method of key intermediate of palbociclib
A palbocicillin and intermediate technology, applied in the field of pharmaceutical preparation, can solve the problems of long synthetic route, inconvenient manufacturers, expensive starting materials, etc., and achieve the effects of short preparation process steps, satisfying use requirements and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
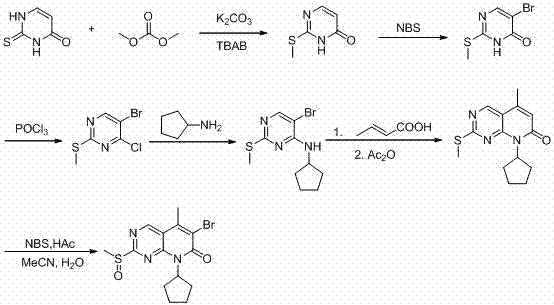
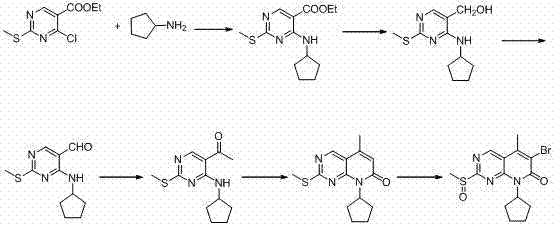
[0024] A kind of preparation method of palbocillin key intermediate, concrete steps are as follows:
[0025] Step 1, the preparation of 2-methylthio-4-pyrimidinone: take 128g of thiouracil, 500g of dimethyl carbonate, 300g of potassium carbonate, 1g of tetrabutylammonium bromide, heat up to 120 ° C for 8h, after the reaction , cooled to room temperature, filtered, the filtrate was decompressed to recover unreacted dimethyl carbonate, the residue was added with 300 ml of water, extracted with 300 ml of ethyl acetate, dried, filtered, and the filtrate was recrystallized with 100 ml of petroleum ether to obtain 132g of white powder , the yield is 93%;
[0026] Step 2, preparation of 5-bromo-2-methylthio-4-pyrimidinone: Take 142 g of 2-methylthio-4-pyrimidinone, dissolve it in 500 ml of dichloromethane, add 190 g of NBS, reflux for 6 hours, After the reaction, cool to room temperature, filter, wash the filtrate with water, dry the organic phase, and recrystallize in an ice bath t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com