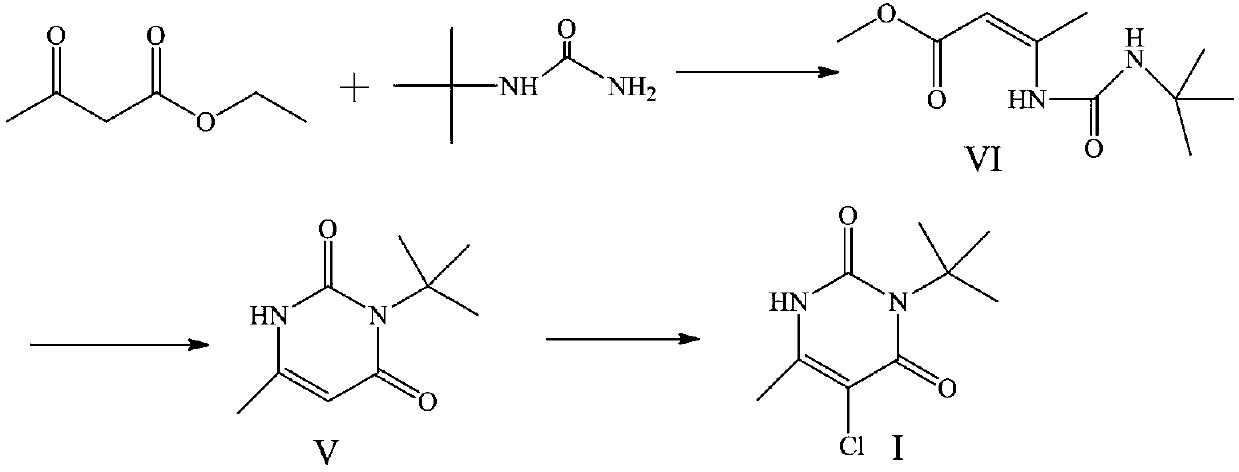
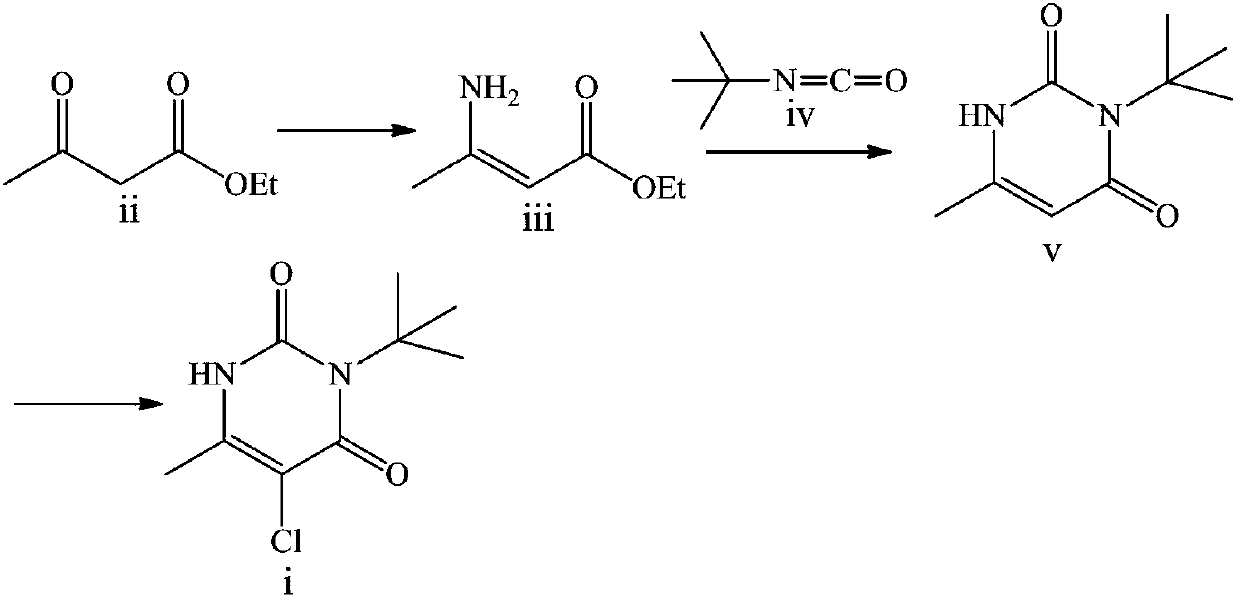
Preparation method of herbicide 3-tert-butyl-5-chloro-6-methyluracil
A technology for terpyridine and herbicide, which is applied in the field of preparation of herbicide terpyridine, can solve the problems of unfriendly environment, many synthetic by-products, and high cost, and achieves avoiding serious by-products, simple and safe operation, and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
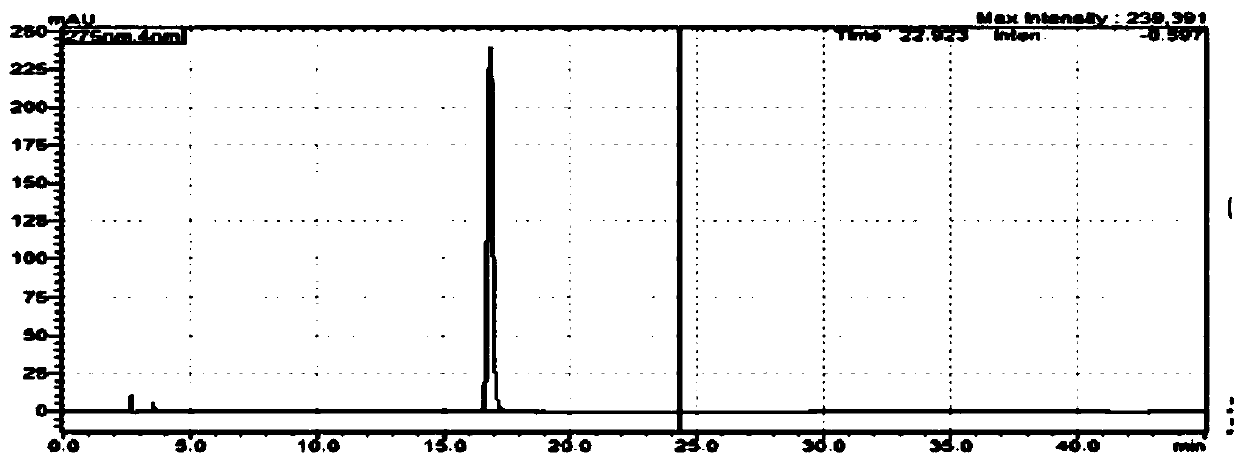
Image
Examples
Embodiment 1
[0025] Weigh 130.1g (1.0mol, 1.0eq) of ethyl acetoacetate into a 500mL three-necked reaction flask, add 84.8g (1.1mol, 1.1eq.) of ammonium acetate, 1.6g (0.01mol, 0.01eq) of iron oxide , 650 mL of absolute ethanol, reacted at 60 ° C for 10 h, and the plate reaction was completed, and the yield of ethyl 3-aminocrotonate was 93.4%.
Embodiment 2
[0027] Weigh 130.1g (1.0mol, 1.0eq) of ethyl acetoacetate into a 500mL three-necked reaction flask, add 77.1g (1.0mol, 1.0eq.) of ammonium acetate, 1.6g (0.01mol, 0.01eq) of iron oxide , 650 mL of absolute ethanol, reacted at 60 ° C for 10 h, and the plate reaction was completed, and the yield of ethyl 3-aminocrotonate was 91.5%.
Embodiment 3
[0029] The ethyl 3-aminocrotonate (0.93mol) obtained in Example 1 was cooled to 30°C, and 100.8g (1,2.0eq) of sodium methoxide, 101.4g (1.02mol, 1.1eq) of tert-butyl isocyanate, and nitrogen were added. Protected and heated to 80°C and refluxed for 5h, cooled down, concentrated to remove ethanol, added 500mL of water for beating, and dried the product to obtain 148.3g of intermediate V with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com