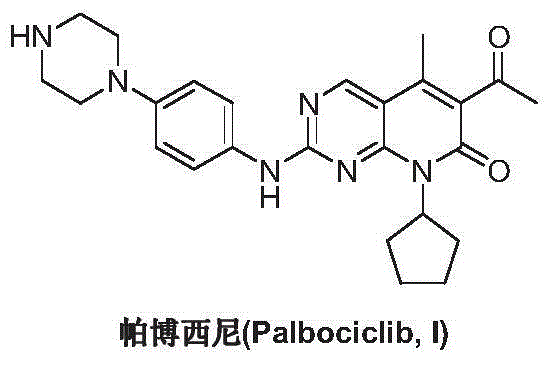
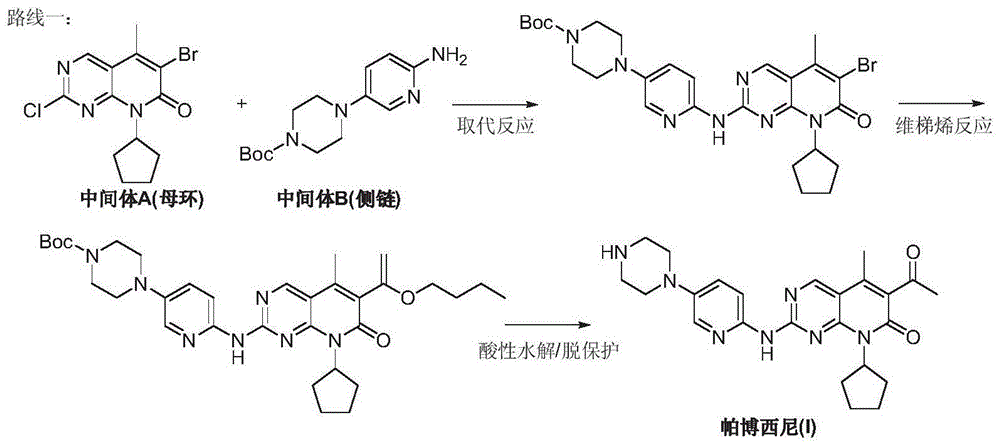
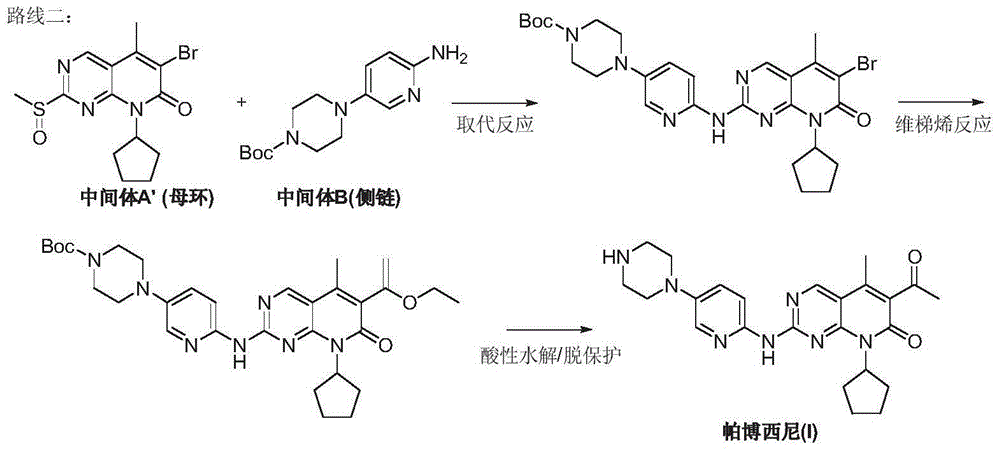
Preparation method for palbociclib
A preparation step, acetyl group technology, applied in the field of preparation of the drug palbociclib, can solve the problems of complex synthesis, rare, complex side reactions, etc., and achieve the effect of economical and environmental protection, simple process, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 2-acetyl-2-butenoic acid methyl ester (II) (7.1g, 50mmol) and methanol 30mL in the dry reaction bottle, add dropwise 30mL methanol solution of sodium methoxide (5.4g, 100mmol) at room temperature, dropwise , and stirred for 15 minutes. A solution of malononitrile (4.0 g, 60 mmol) in 20 mL of methanol was added dropwise. The temperature was raised to reflux of methanol, and the reaction was continued for 4-5 hours. TLC detected that the reaction was complete. The solvent was recovered under reduced pressure, and the residue was dissolved in water. The pH value of the solution was adjusted to 8.0-9.0 with dilute hydrochloric acid under ice-cooling, extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated, and the obtained residue was washed with ethyl acetate and n-hexane (1:1 , V / V) recrystallized and dried in vacuo to give white solid 1,4,5,6-tetrahydro-2-methoxy-4-methyl-5-acetyl-6-oxo-3-pyrid...
Embodiment 2
[0036]Add 1,4,5,6-tetrahydro-2-methoxy-4-methyl-5-acetyl-6-oxo-3-pyridinecarbonitrile (III) (2.1g, 10mmol) into the reaction flask , 0.6g of silicone oil containing 60% sodium hydride and 30mL of N,N-dimethylformamide, heated to 55°C, and stirred for 30 minutes. After cooling down to room temperature, iodocyclopentane (2.9 g, 15 mmol) was added, the temperature was raised to 55°C again, and the reaction was stirred for 30 minutes. TLC detects that the reaction is complete. Quench the reaction with water, extract three times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, concentrate, and a solid precipitates out, the resulting crude product is recrystallized with n-hexane and ethyl acetate (2:1, V / V), and dried in vacuo The off-white solid N-cyclopentyl-1,4,5,6-tetrahydro-2-methoxy-4-methyl-5-acetyl-6-oxo-3-pyridinecarbonitrile (IV) 2.1 g, yield 76.1%; mass spectrum (EI): m / z 277 (M+H).
Embodiment 3
[0038] In a nitrogen atmosphere, add N-cyclopentyl-1,4,5,6-tetrahydro-2-methoxy-4-methyl-5-acetyl-6-oxo-3-pyridylmethyl to the reaction flask Nitrile (IV) (2.8g, 10mmol), N-[5-(1-piperazinyl)-2-piperidinyl]guanidine (V) (4.4g, 20mmol) and xylene 15mL, heated to 150°C, Stir the reaction for 18-20 hours, and TLC detects that the reaction is complete. The solvent was distilled off under reduced pressure, cooled to room temperature, methanol was added, and a solid precipitated out. Filter, wash the filter cake twice with cold methanol, and dry in vacuo to obtain off-white solid 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridine Base]amino]-5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-one (VI) 2.56g, yield 58.2%; mass spectrum (EI): m / z 450 (M +H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com