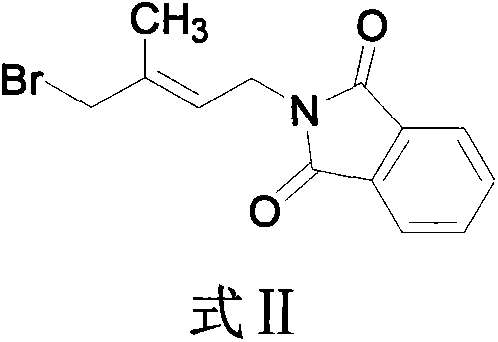
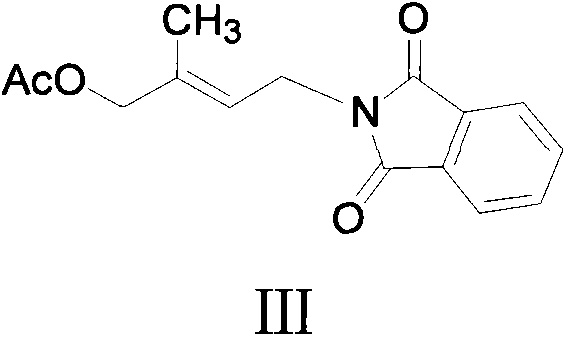
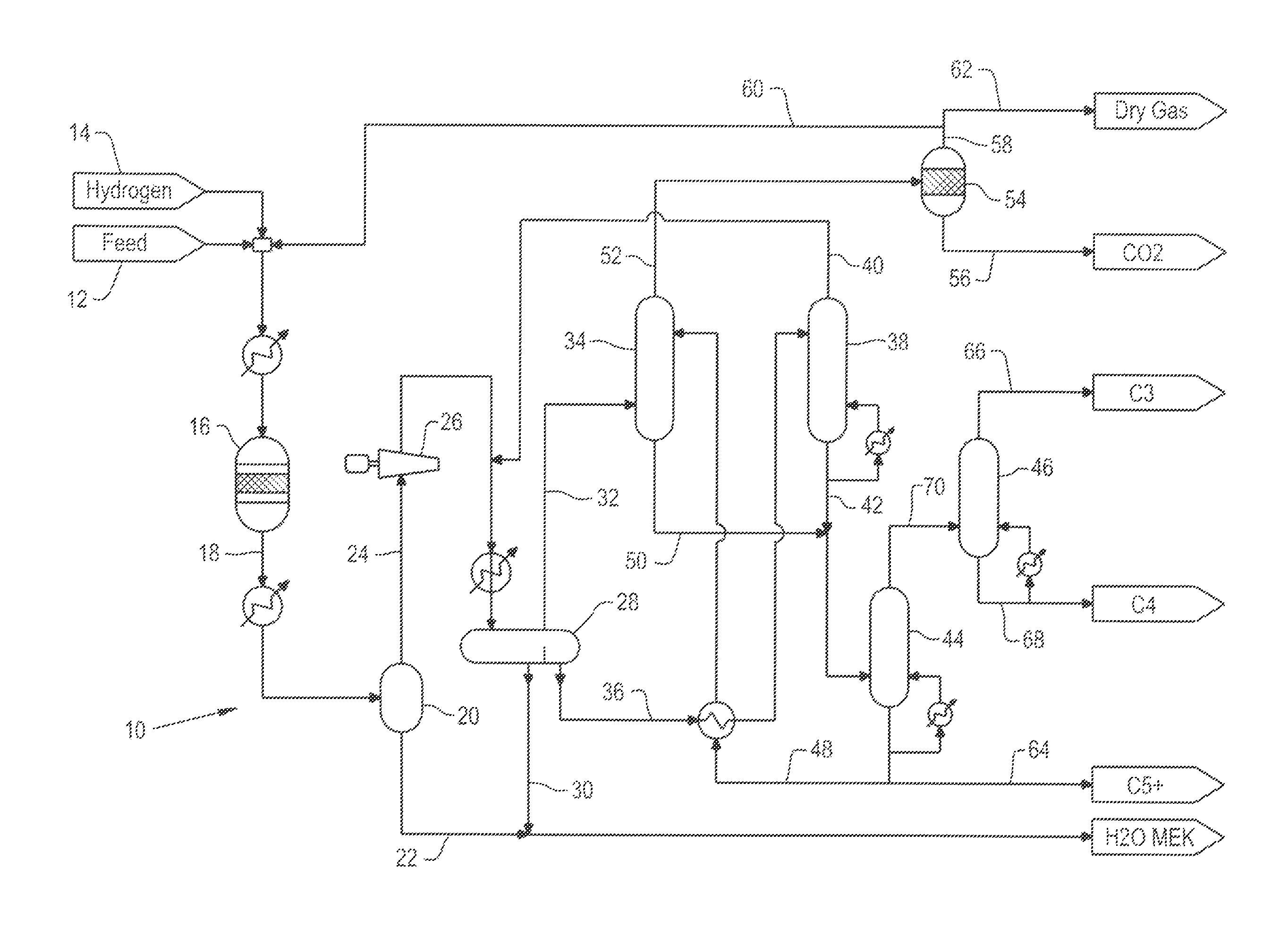
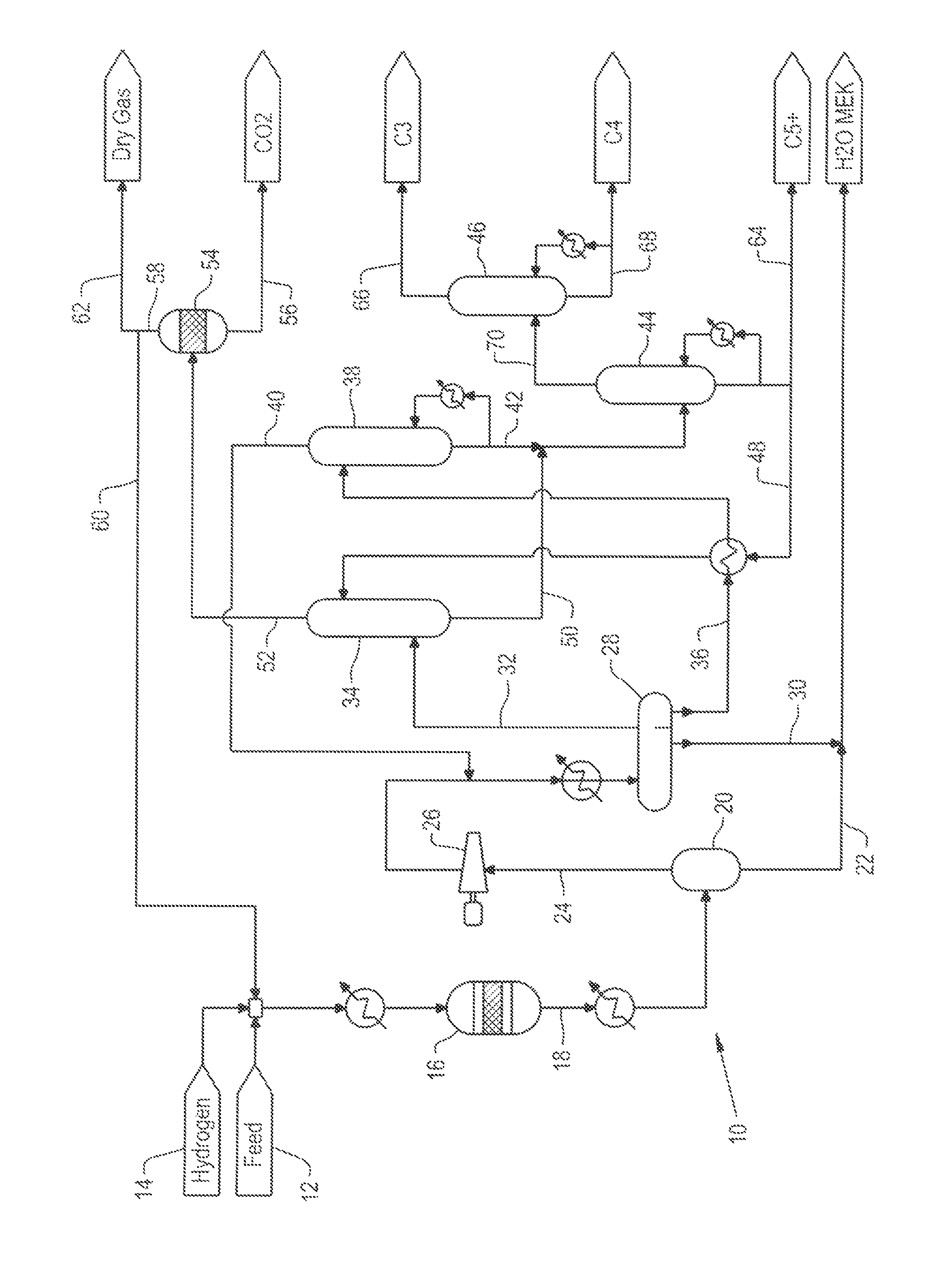
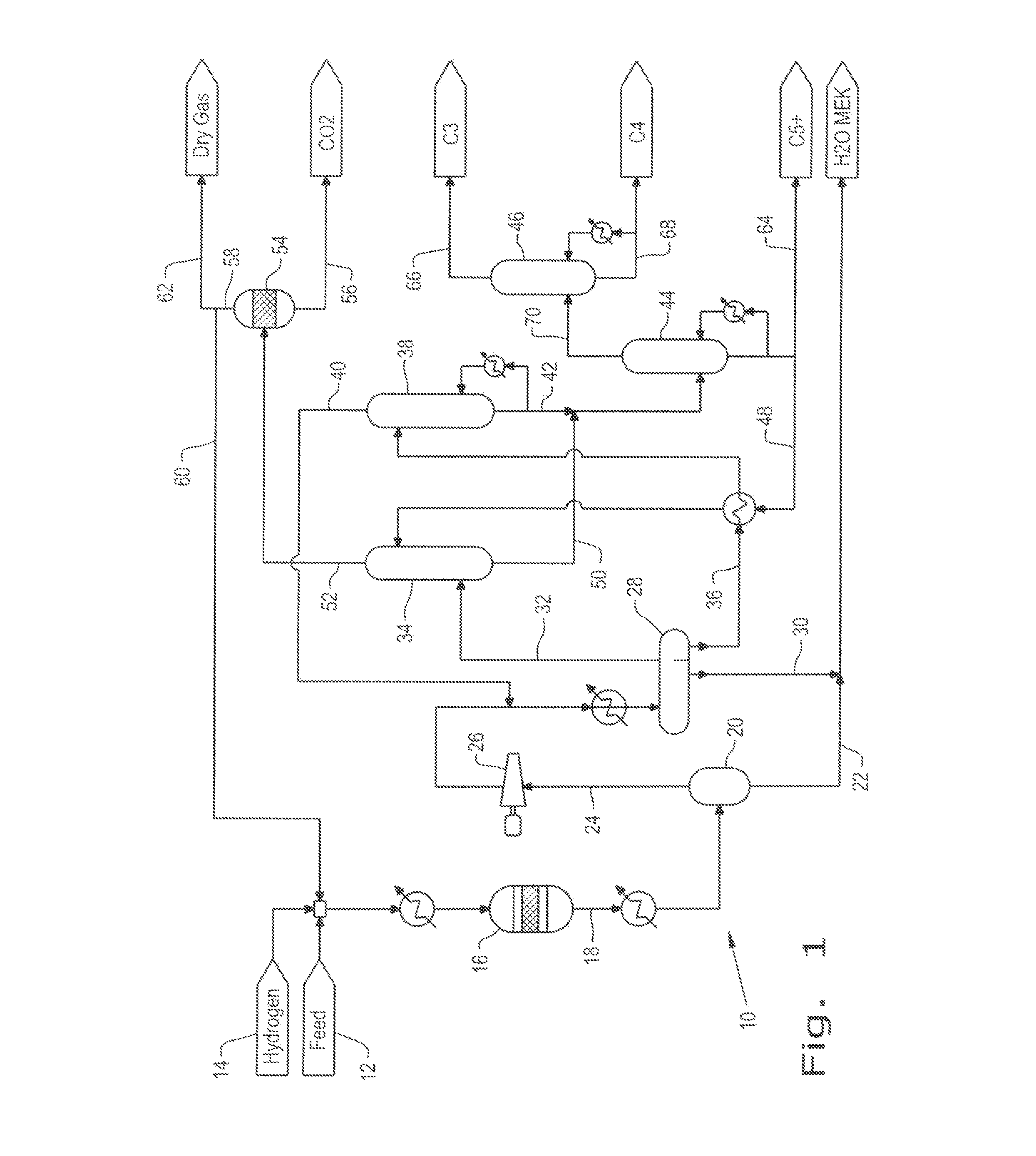
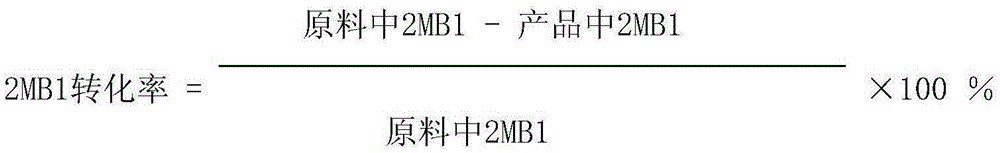
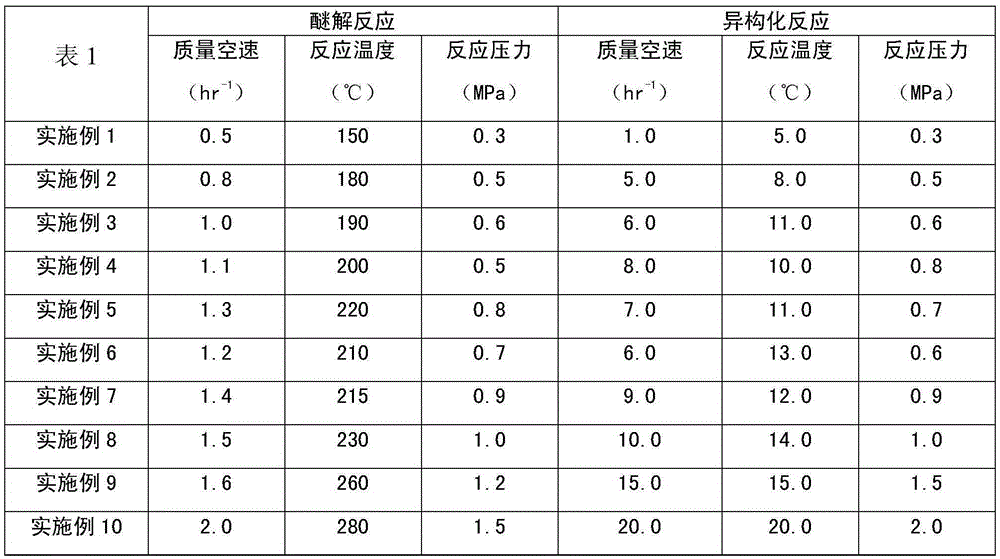
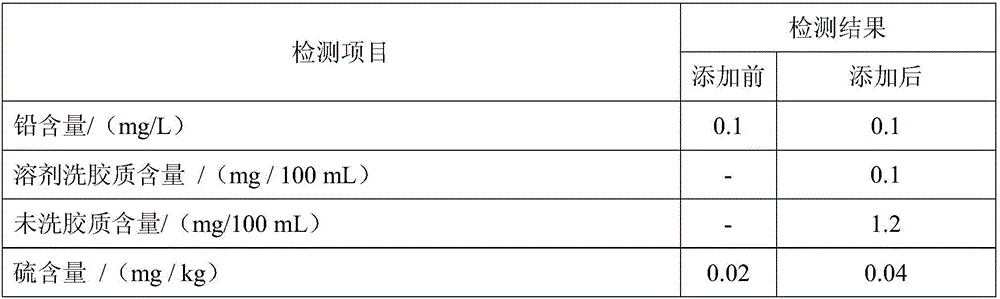
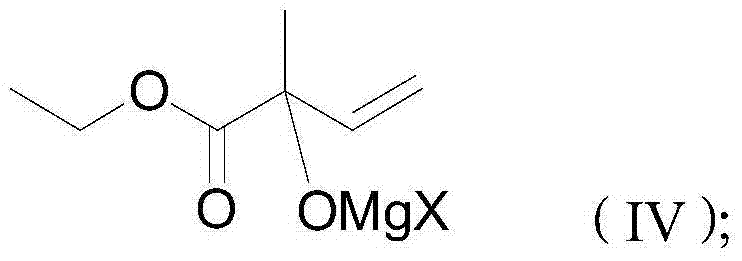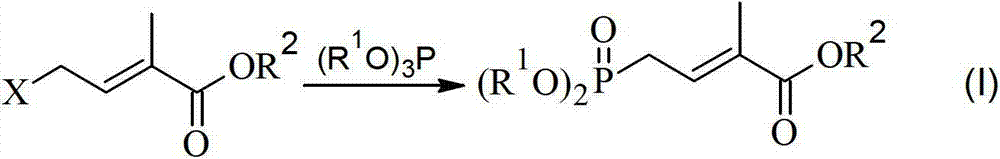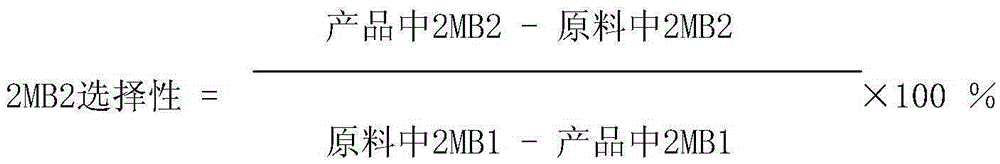Patents
Literature
57 results about "2-Methyl-2-butene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
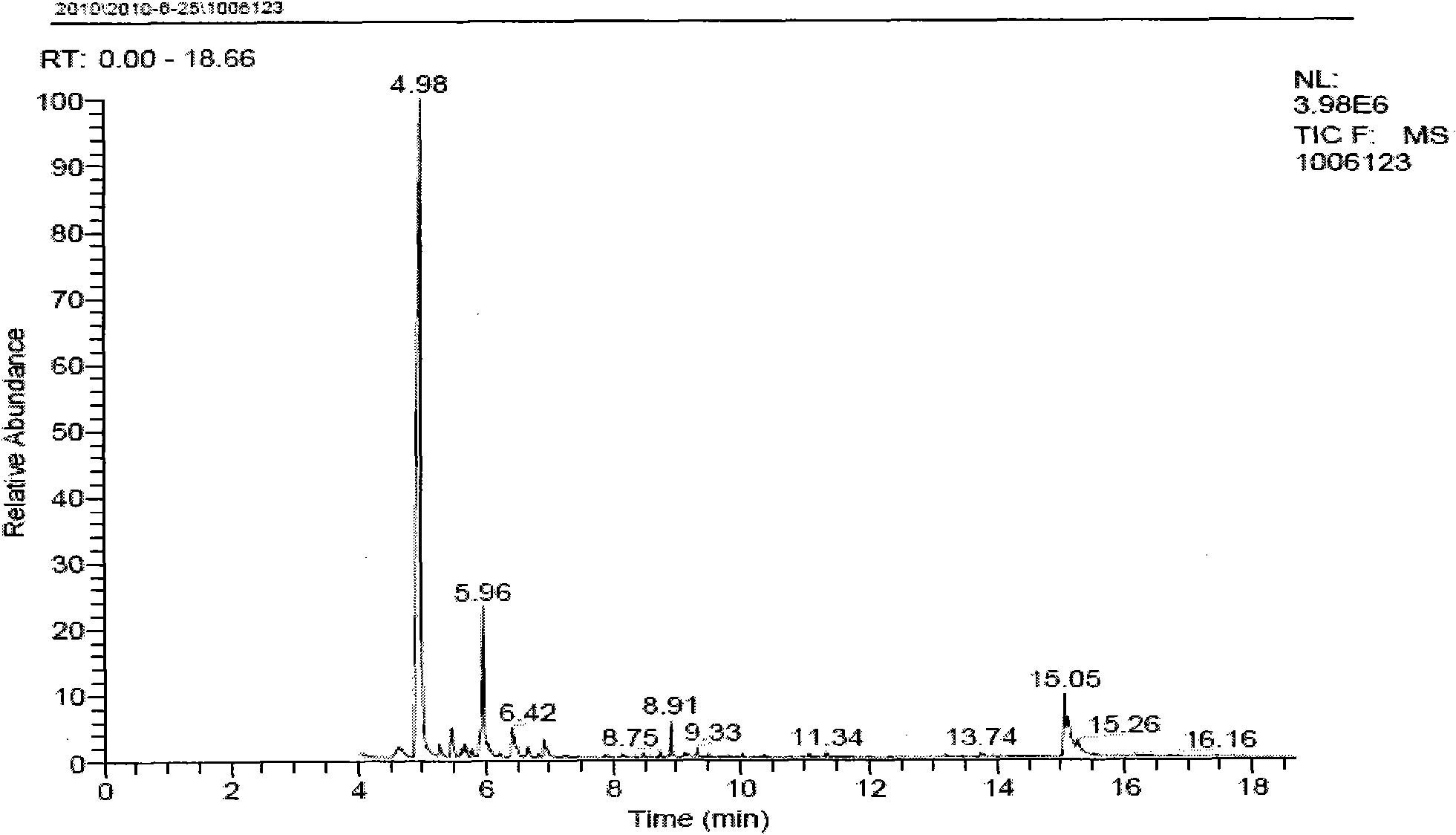
2-Methyl-2-butene, 2m2b, 2-methylbut-2-ene, also amylene is an alkene hydrocarbon with the molecular formula C₅H₁₀. Used as a free radical scavenger in trichloromethane (chloroform) and dichloromethane (methylene chloride).
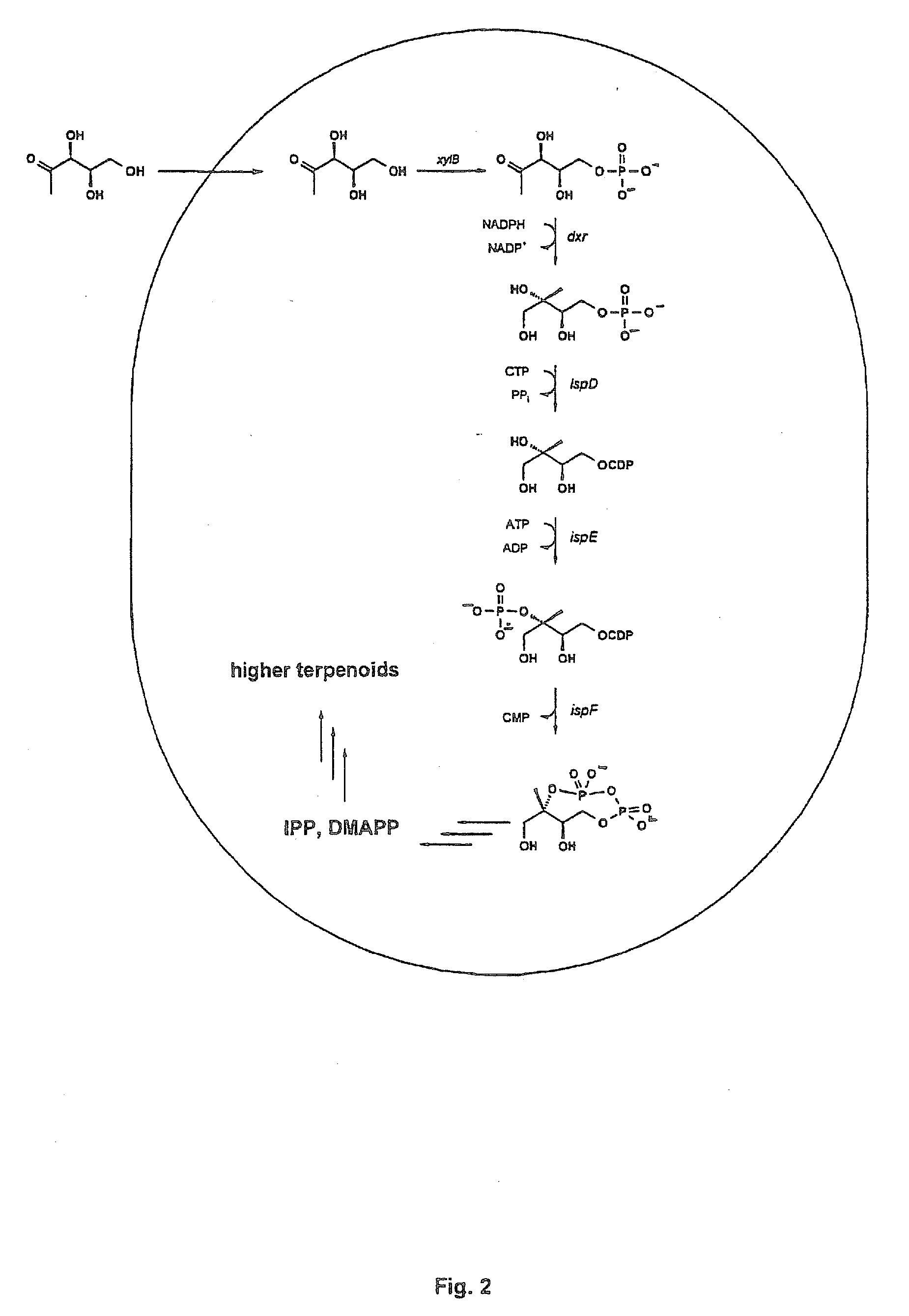
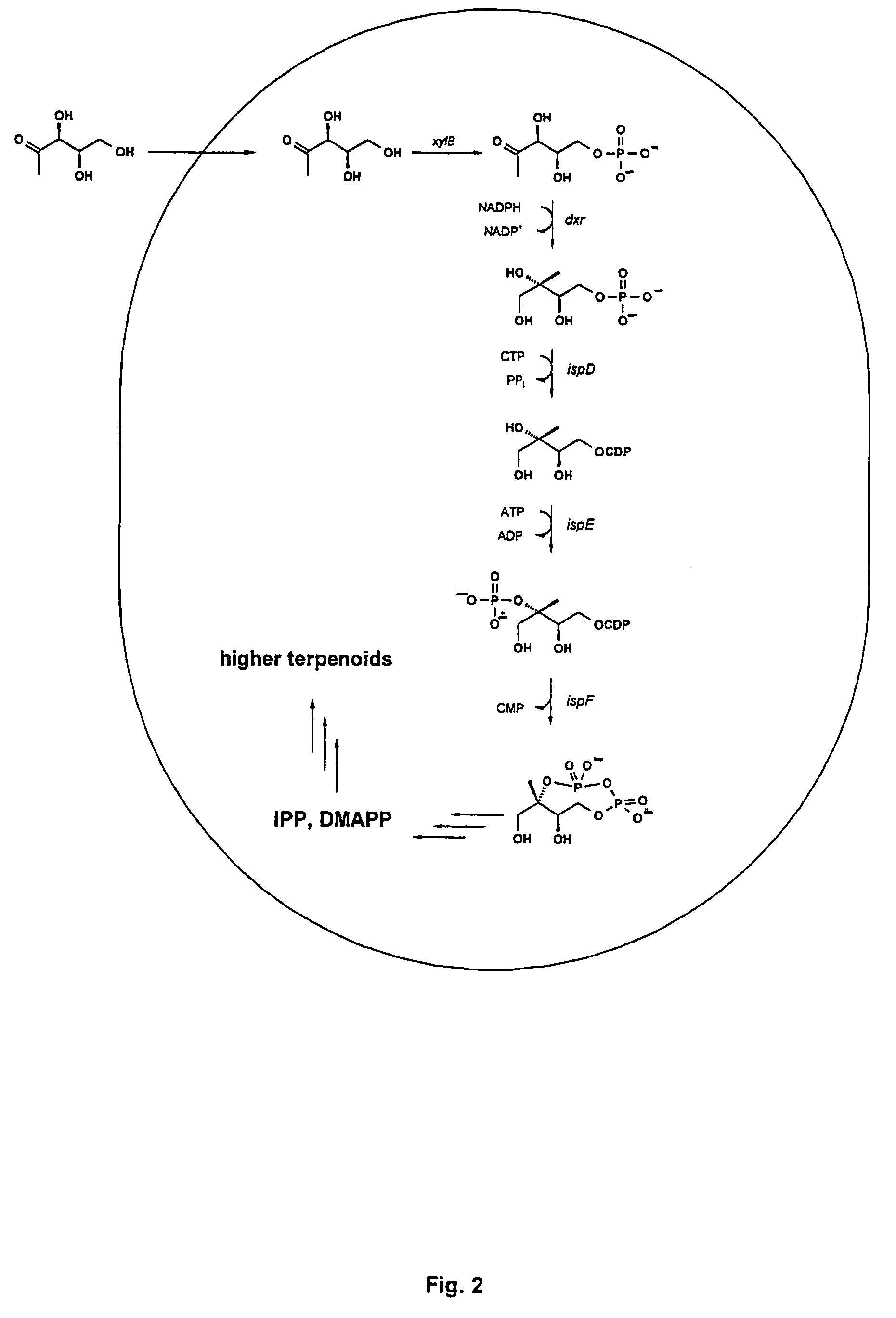
Intermediates and enzymes of the non-mevalonate isoprenoid pathway
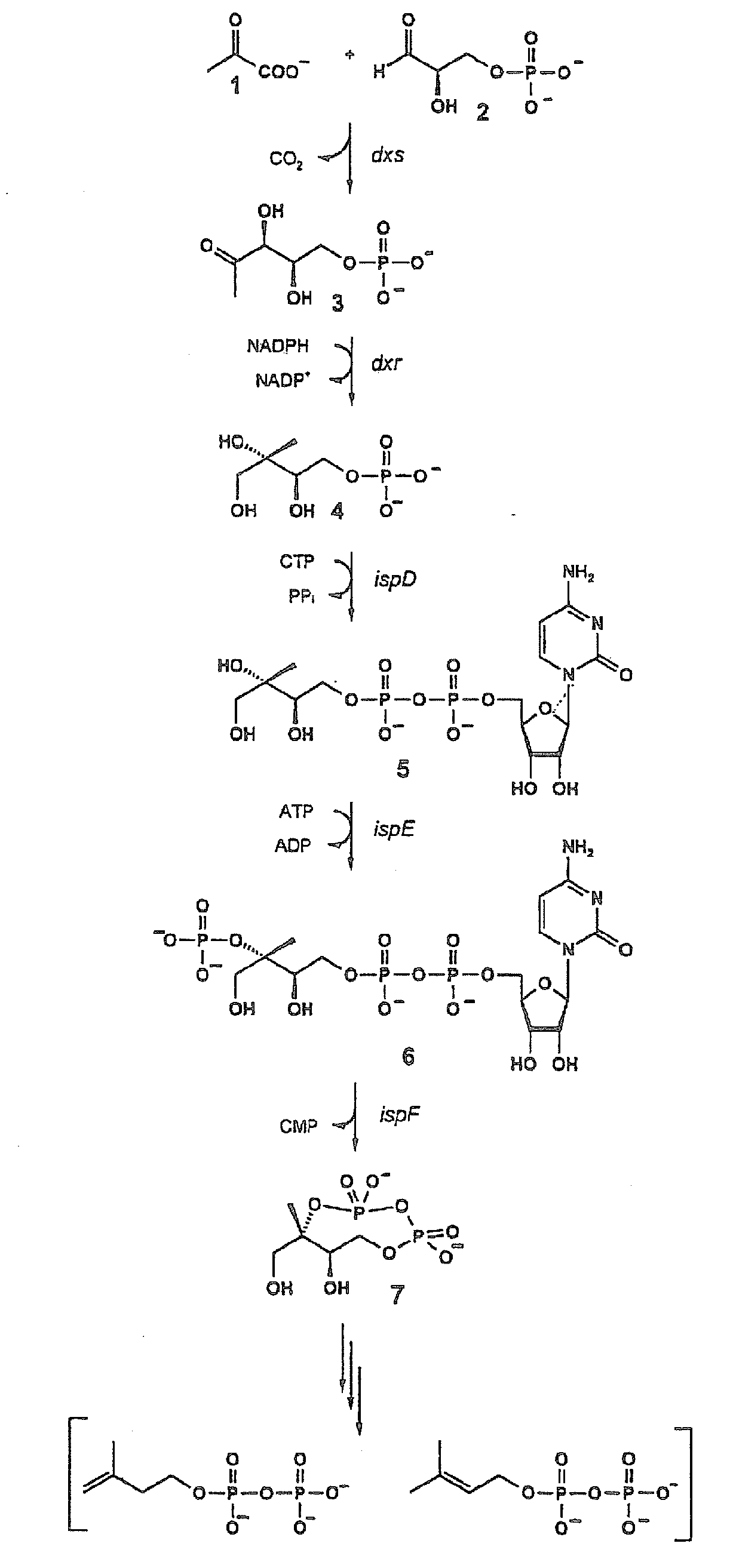
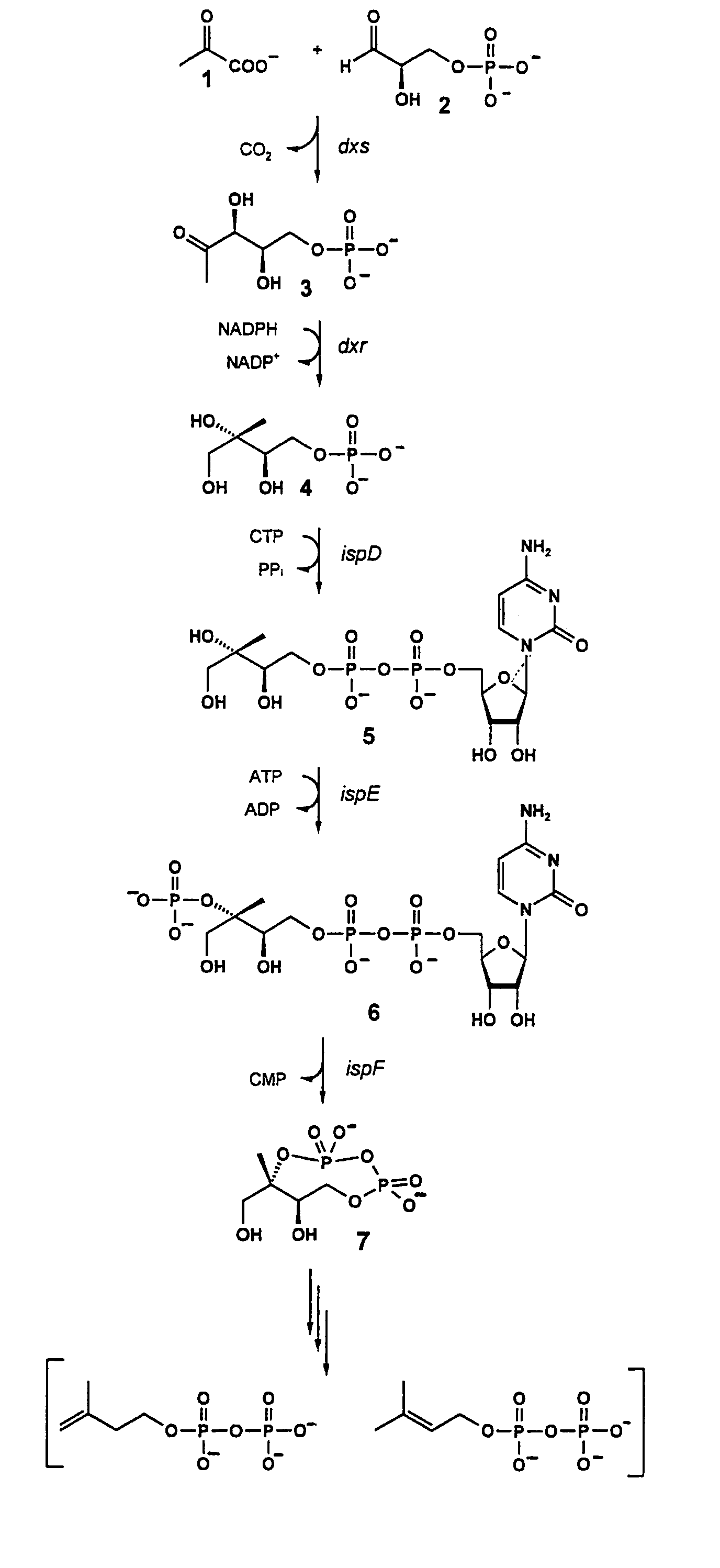
The invention provides a protein in a form that is functional for the enzymatic conversion of 2C-methyl-D-erythritol 2,4-cyclodiphosphate to 1-hydroxy-2-methyl-2-butenyl 4-diphosphate notably in its (E)-form of the non-mevalonate biosynthetic pathway to isoprenoids. The invention also provides a protein in a form that is functional for the enzymatic conversion of 1-hydroxy-2-methyl-2-butenyl 4-diphosphate, notably in its (E)-form, to isopentenyl diphosphate and / or dimethylallyl diphosphate. Further, screening methods for inhibitors of these proteins are provided. Further, 1-hydroxy-2-methyl-2-butenyl 4-diphosphate is provided and chemical and enzymatic methods of its preparation.
Owner:BACHER ADELBERT +5
Method for modifying covalent organic framework material
ActiveCN109942827ANo pollution in the processImprove enrichment capacityPeptide preparation methodsAcetic acidBenzene
The invention provides a method for modifying a covalent organic framework material. The method comprises the following steps: (1) mixing 1,3,5-tris-(4-aminophenyl)benzene with 2,5-dioxymethyl-terephthalaldehyde, adding o-dichlorobenzene / n-butanol and an acetic acid catalyst, carrying out freezing-exhausting-unfreezing circulation for several times, carrying out flame sealing, heating, centrifugally collecting precipitates, washing, carrying out Soxhlet extraction, drying, and collecting powder, so as to obtain TPB-DMTA-COF; (2) adding 2-methyl-2-butene, a sodium chlorite water solution and glacial acetic acid into dioxane suspension liquid of the TPB-DMTA-COF powder, and stirring in a dark environment, so as to obtain purple solids; and (3) re-oxidizing the purple solids by virtue of thesodium chlorite water solution, and carrying out filtration separation, washing, drying, so as to obtain purple powder. The preparation method is simple, the synthesis conditions are easily controlled, and prepared O-TPB-DMTA-COF powder has a good glycopeptides enriching effect.
Owner:WUHAN UNIV OF TECH
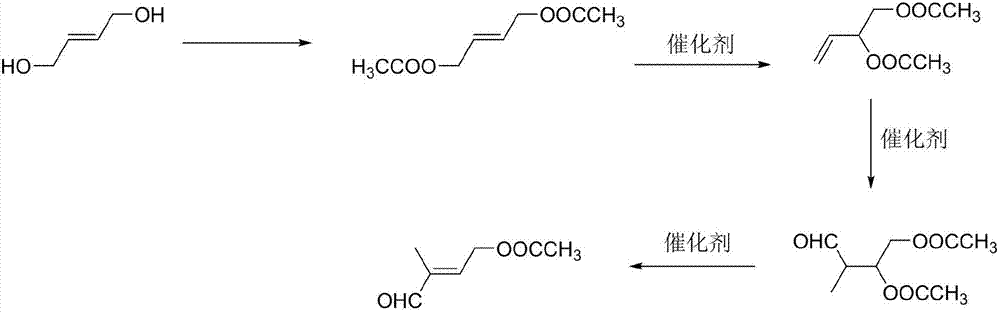
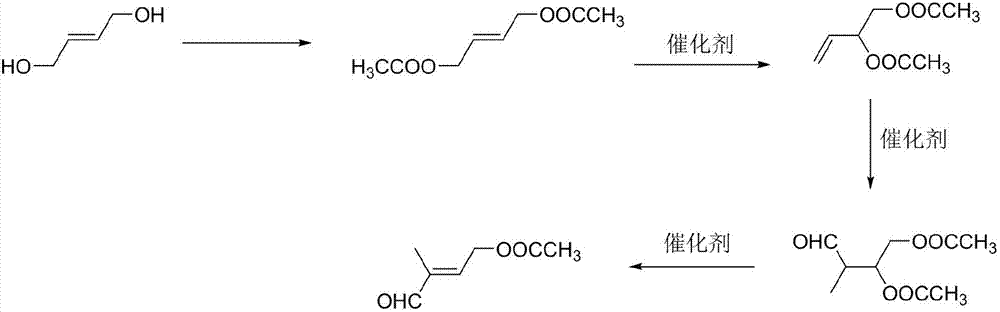
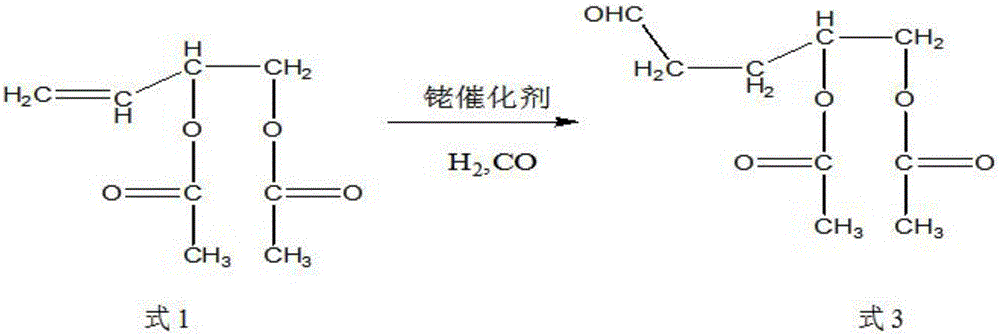
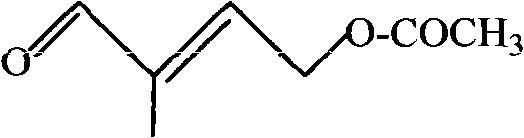
Preparation method of 4-acetoxyl-2-methyl-2-butene-1-aldehyde
InactiveCN107286017AIncrease productionReduce lossesOrganic compound preparationCarboxylic acid esters preparationIsomerizationOrganic synthesis
The invention discloses a preparation method of 4-acetoxyl-2-methyl-2-butene-1-aldehyde and relates to the technical field of organic synthesis. The method comprises the steps of firstly reacting 1,4-butylene glycol serving as a raw material with an esterification reagent to prepare 1,4-butylene glycol diethyl ester; preparing 3,4-diacetoxy-1-butene through isomerization reaction; carrying out hydroformylation reaction on carbon monoxide and hydrogen to prepare 2-methyl-3,4-diacetoxy-1-butyraldehyde; and finally preparing the 4-acetoxyl-2-methyl-2-butene-1-aldehyde through elimination reaction. The method is simple in reaction operation, cheap and available in starting material, high in conversion rate and low in catalyst dosage, thereby effectively reducing the synthesis cost.
Owner:安徽智新生化有限公司
Method for preparing 4-acetoxy-2-methyl-2-butene-1-aldehyde
InactiveCN102311339AEasy to rearrangeEmission reductionOrganic compound preparationCarboxylic acid esters preparation2-Methyl-2-buteneMethyl group
The invention discloses a method for preparing 4-acetoxy-2-methyl-2-butene-1-aldehyde. The method is characterized by comprising the following steps: firstly, hydrolyzing 2-methyl-2-acetoxy-1,1-dimethoxy-3-butene to obtain 2-methyl-2-acetoxy-3-butene-1-aldehyde; and then, carrying out rearrangement reaction on the obtained 2-methyl-2-acetoxy-3-butene-1-aldehyde to obtain 4-acetoxy-2-methyl-2-butene-1-aldehyde. The method disclosed by the invention has the advantages of concise route, mild reaction system and high product content and yield.
Owner:SHAOXING UNIVERSITY +1
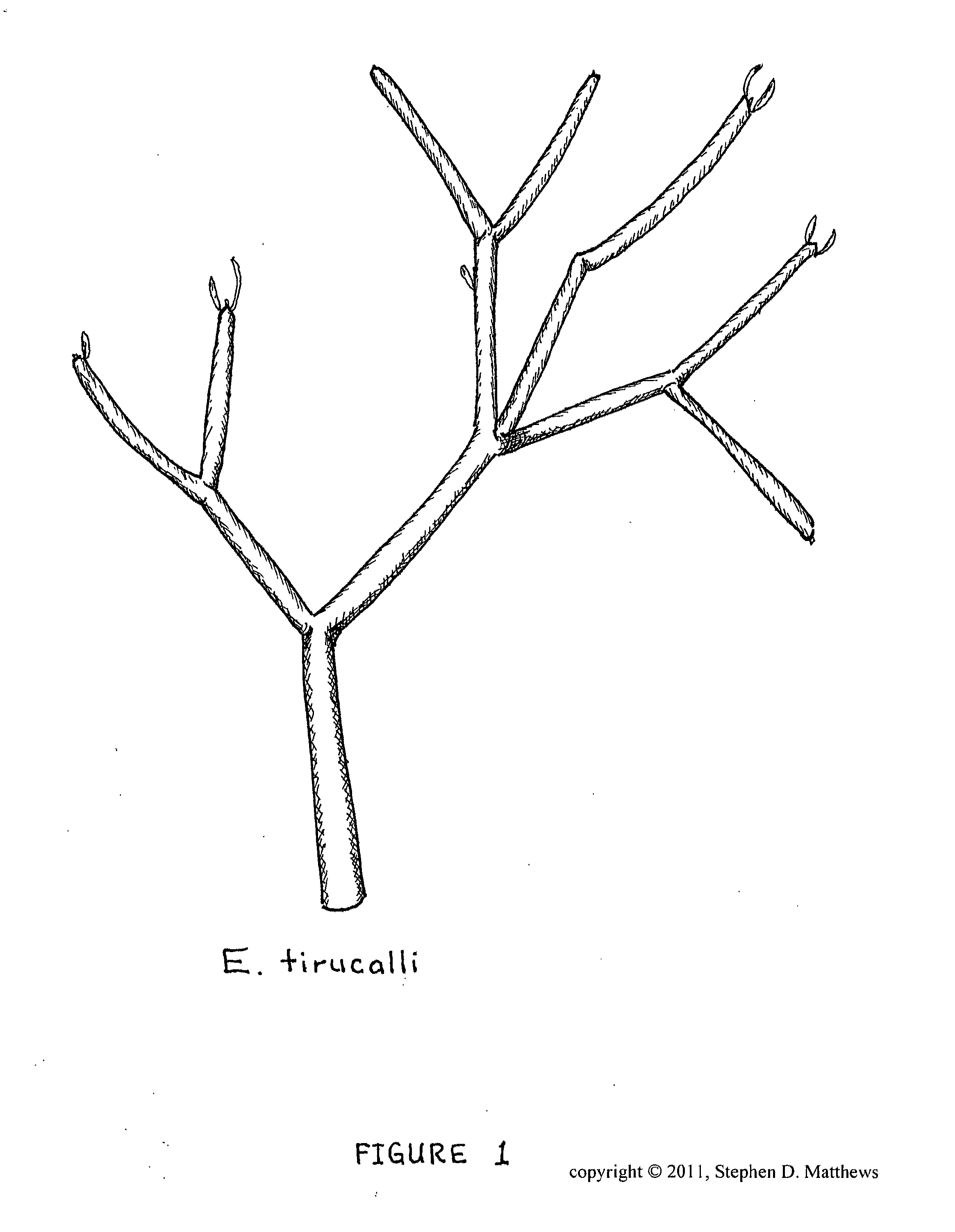
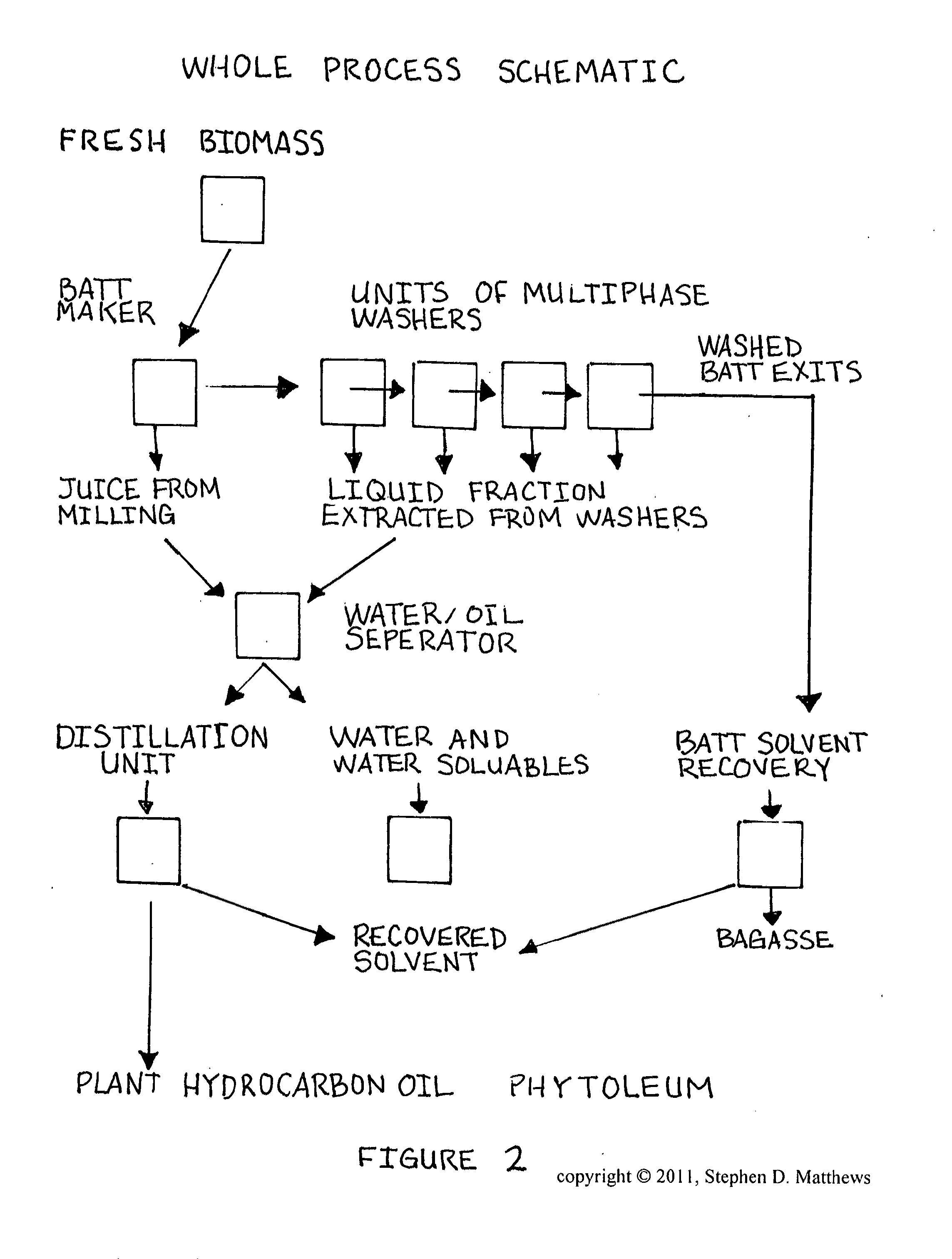
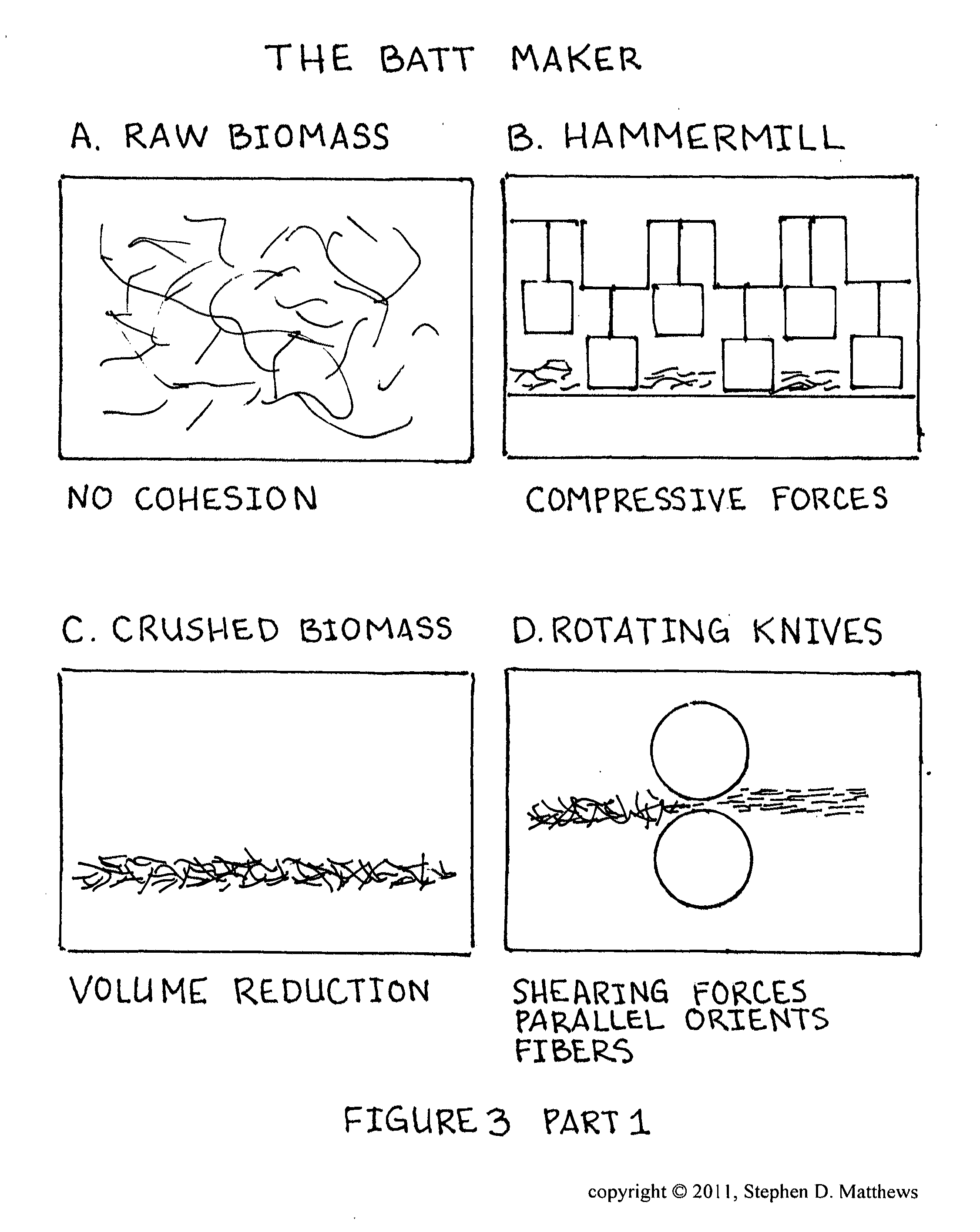
Composition of matter comprising of the creation of a low molecular weight hydrocarbon fluid exhibiting mainly oligomerized pentenes mainly comprised of 2-Methyl-2-Butene subunits as well as related plant isoprenoids composed of 2-Methyl-1-Butene subunits and other hydrocarbons from Euphorbia tirucalli biomass and a process for the extraction and refinement in making the same composition through the creation of solvent permeable batting mat and a multi-phase solvent extraction
InactiveUS20120197052A1Efficient extraction and refinementLiquid carbonaceous fuelsLiquid hydrocarbon mixture productionFiberMethyl group
A composition of matter with of the creation of low molecular weight hydrocarbon fluid called Phytoleum from Euphorbia tirucalli biomass and a process for the extraction and refinement in making the same composition through the creation of a batting mat and multi-phase solvent extraction. A preferred embodiment includes the steps of manufacturing a fibrous batting mat from the raw biomass, crushing the biomass, shearing the biomass with a rotating knives blade array, compressing the biomass by passing the biomass through press rollers, amalgonating the biomass into a Batting Mat, subjecting the Batting Mat to a phased multi-wash solvent system, extracting the solvents and oils liquid solution for recovery, subjecting the liquid solution to a centrifugation system to extract the Phytoleum hydrocarbon oil from the other components, and refining the final product to yield Phytoleum which is a composition of matter including Tirucallene A and Tirucallene B and other oligomerized pentenes.
Owner:MATTHEWS STEPHEN DANIEL
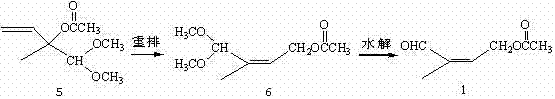
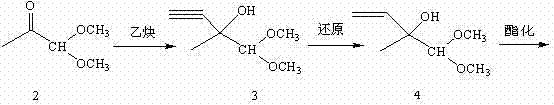
4-halogenated-2-methyl-2-ethyl crotonate preparing method
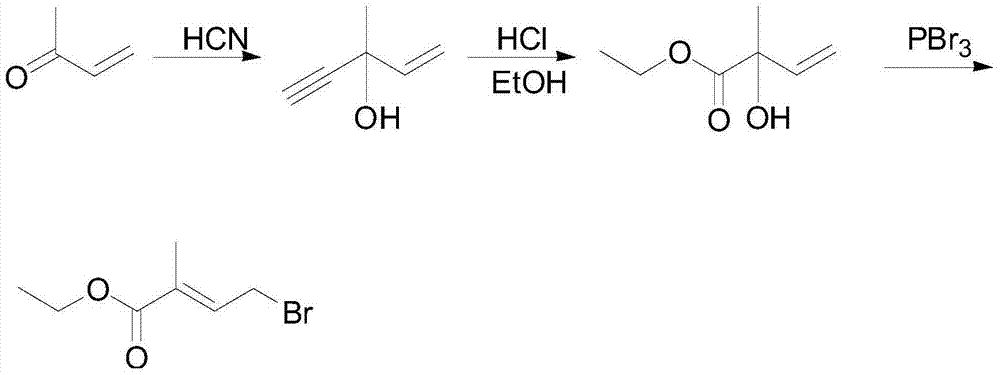
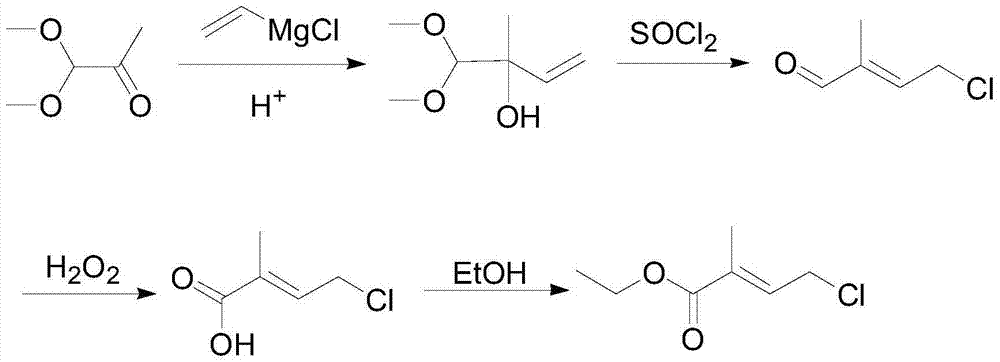
ActiveCN104513164ASimple processRaw materials are easy to getOrganic compound preparationCarboxylic acid esters preparationGrignard reagent2-Methyl-2-butene
The invention discloses a 4-halogenated-2-methyl-2-ethyl crotonate preparing method which includes following steps: (1) performing a Grignard reaction to ethyl pyruvate (II) and a vinyl magnesium halide Grignard reagent (III) to obtain a condensation compound (IV) after the reaction finished, and carrying out a hydrolysis reaction to the condensation compound (IV) to obtain a hydroxyl compound (V); and (2) performing a halogenating reaction to the hydroxyl compound and a haloid acid to obtain the 4-halogenated-2-methyl-2-ethyl crotonate (I). The 4-halogenated-2-methyl-2-ethyl crotonate preparing method is simple in total technology, allows raw materials to be obtained easily, is good in reaction selectivity, is less in by-products and is high in yield. The employed raw materials are all low-toxic or toxic-free and are all corrosiveness-free so that the method is not rigorous in requirement of devices, is environmental-friendly and is suitable for industrialized production.
Owner:SHANGYU NHU BIOCHEM IND +2
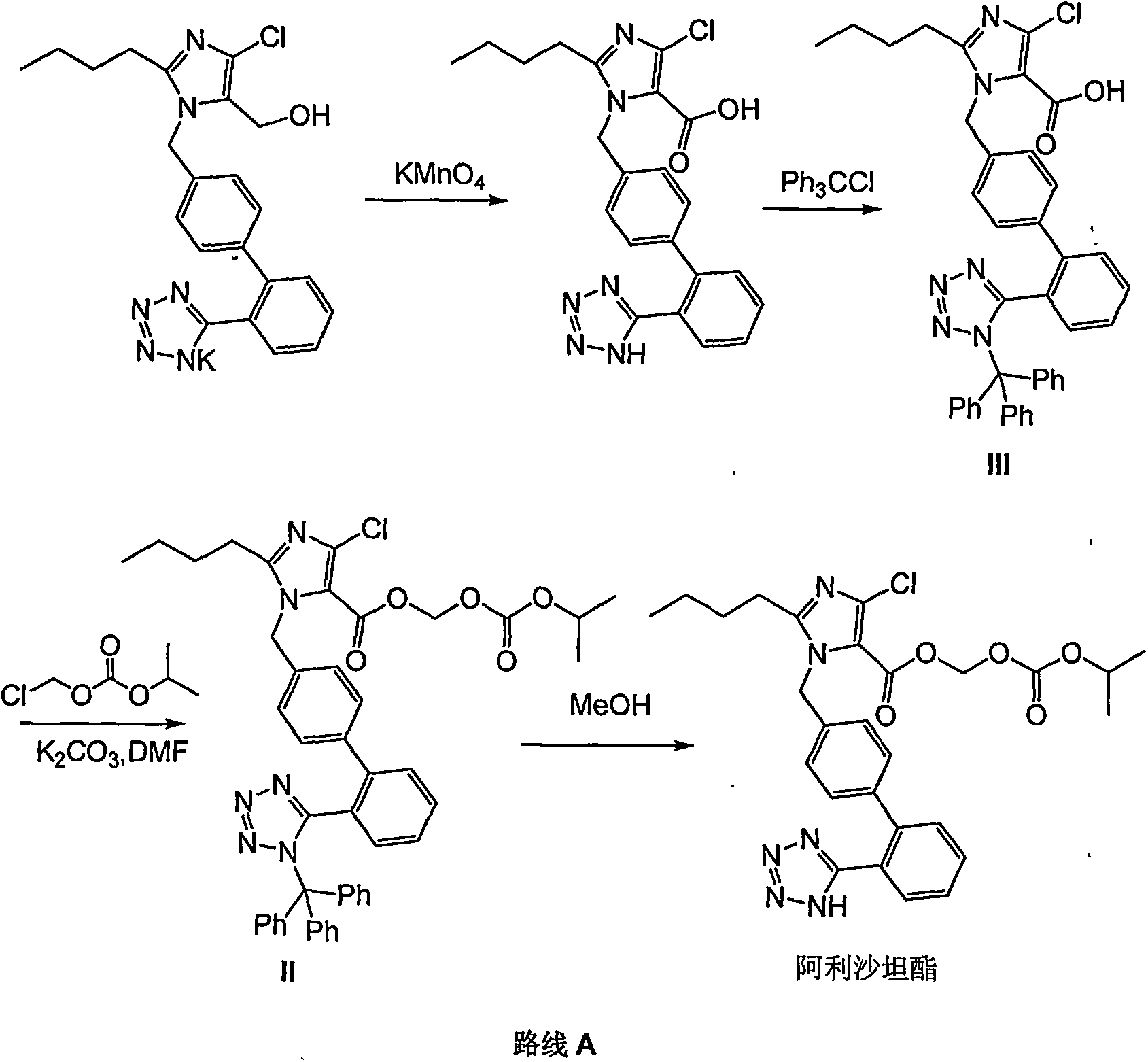
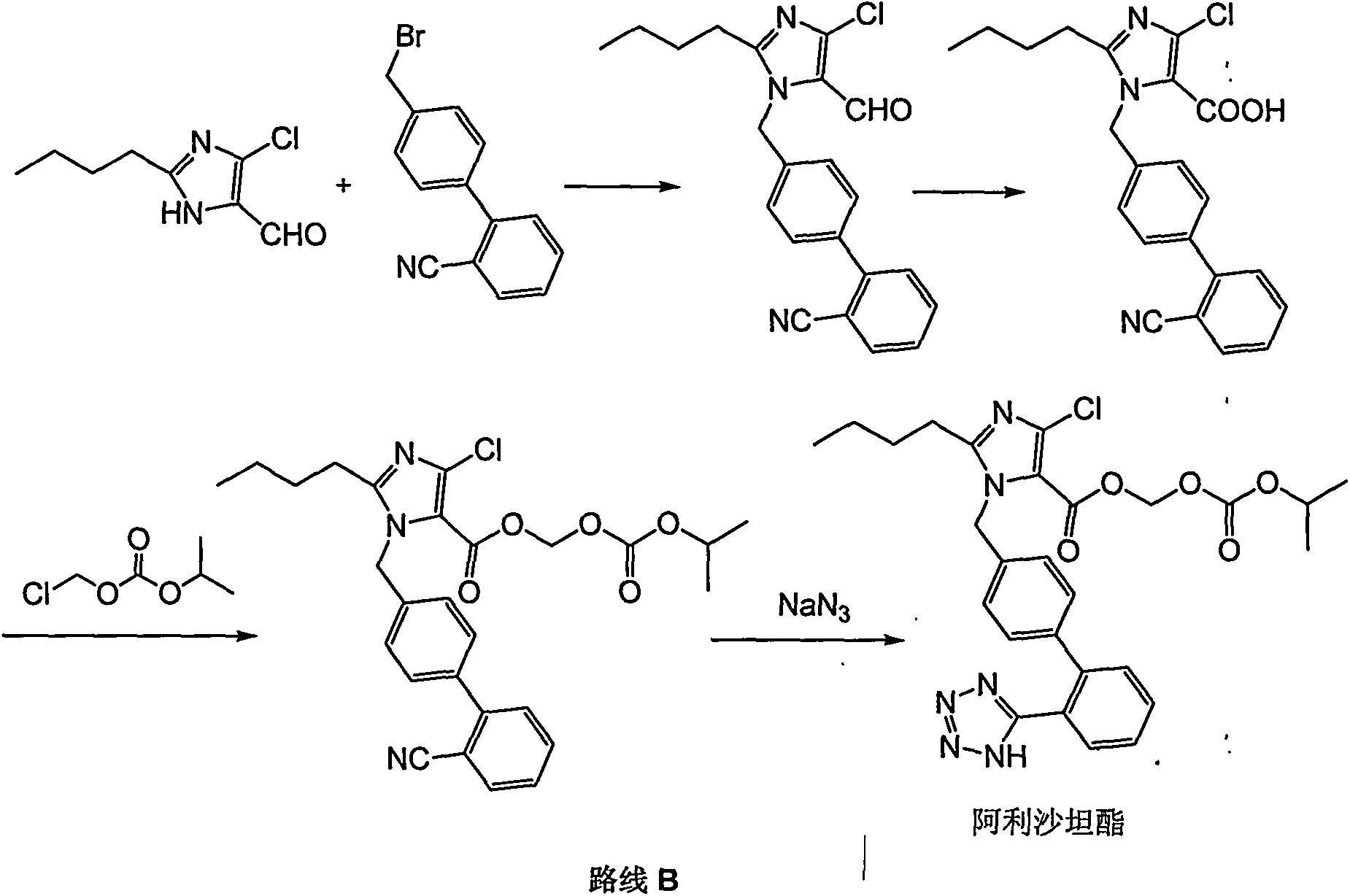
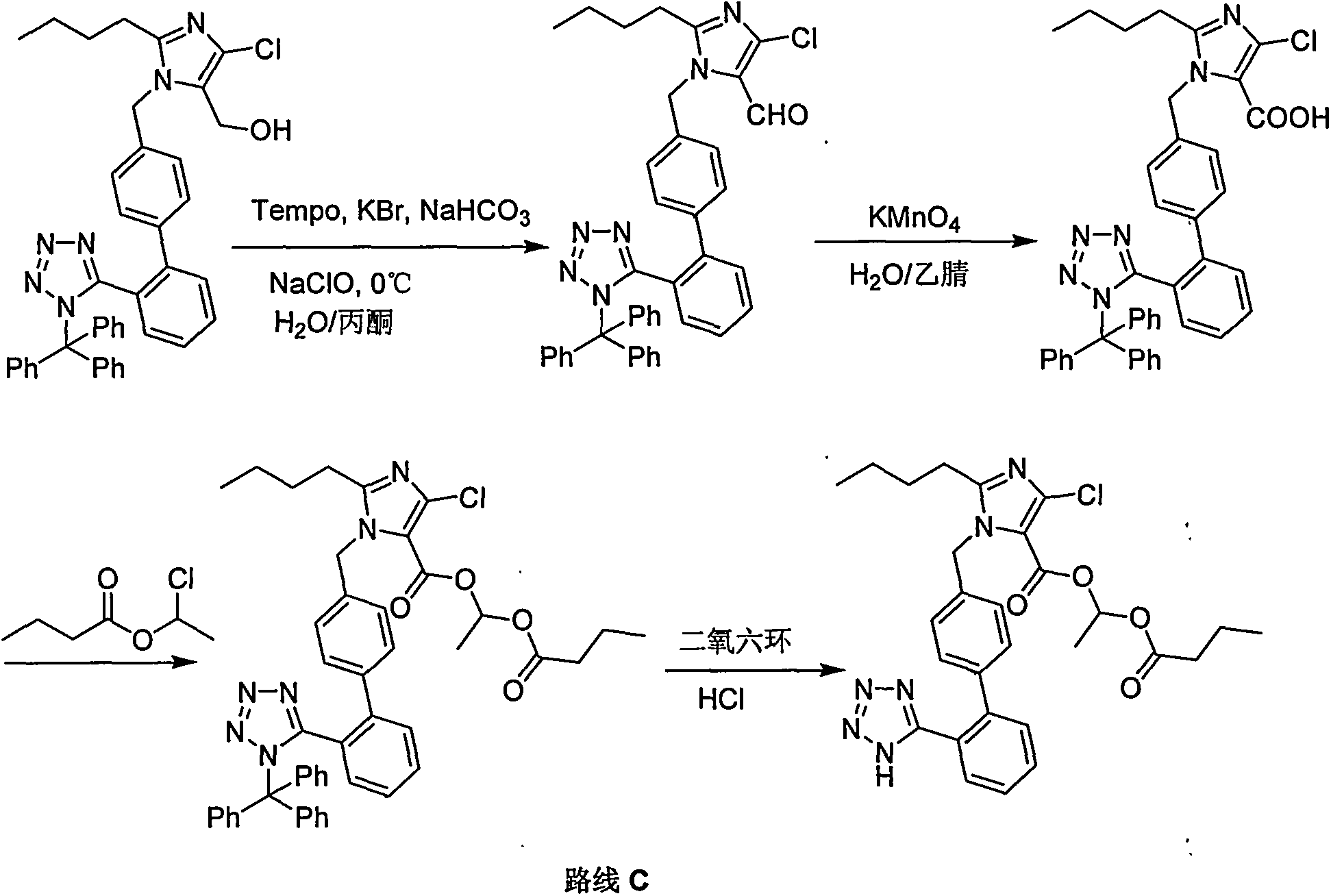
Preparation method for Allisartan Isoproxil
The invention relates to a preparation and refining method for Allisartan Isoproxil. The preparation method comprises the following steps: trityl chloride losartan is oxidized in a polarity organic solvent at the presence of a catalyst to obtain a compound expressed in formula IV; the compound expressed in the formula IV is further oxidized by oxidation reagent hydrogen peroxide / sodium chlorite, Resorcino / sodium chlorite, sulfamic acid / sodium chlorite or 2-methyl-2-butene / sodium chlorite to obtain Allisartan Isoproxil intermediate expressed in formula III; then the Allisartan Isoproxil intermediate is subjected to ester formation and deprotection to obtain an Allisartan Isoproxil crude product; finally, the Allisartan Isoproxil crude product is refined by ethanol / normal heptane to obtain purified Allisartan Isoproxil. According to the preparation method for Allisartan Isoproxil, the yield and efficiency are high, the by-product is few, the preparation method is environmental-friendly and suitable for industrialized production, the operation is simple and safe and the cost is low.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Separation method of piperylene
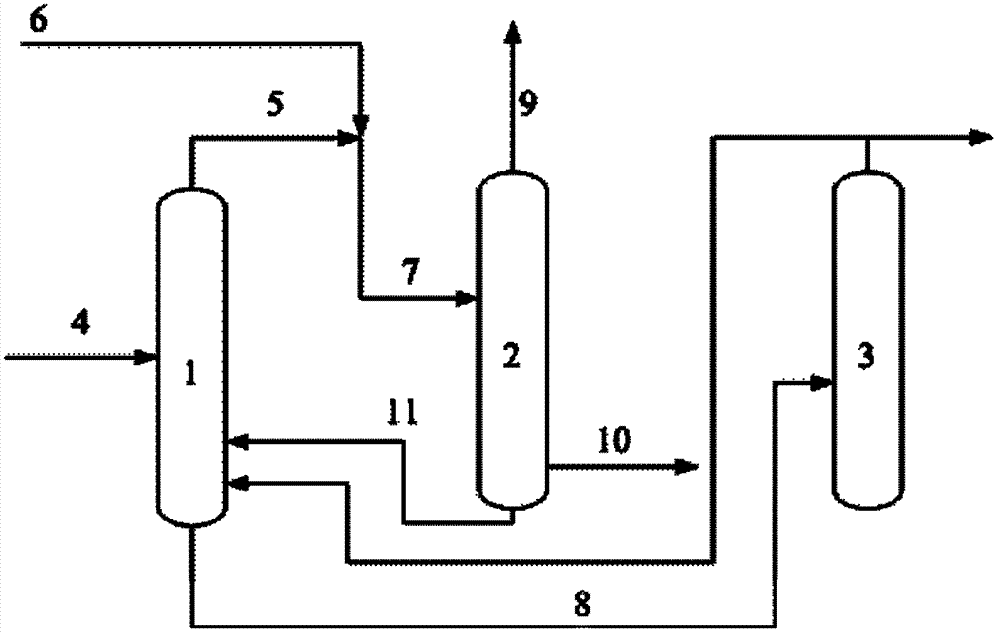
ActiveCN103086822AReduce the number of theoretical boardsReduce the reflux ratioDistillation purification/separation2-Methyl-2-buteneFlow ratio
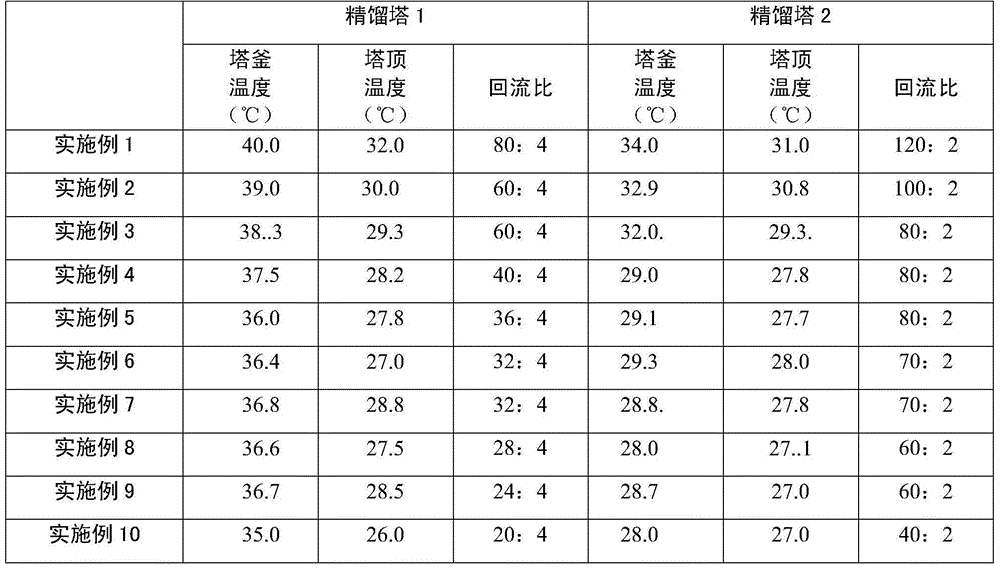
The invention discloses a piperylene separation method. According to the invention, piperylene is separated through azeotropic rectification. An azeotropic material flow is introduced into a fed material of a piperylene tower. The azeotropic material flow comprises n-pentane and 2-methyl-2-butene. Flow ratio of the azeotropic material flow to a C5 removing tower top recovered material flow is 0.01-1. The two are mixed and are delivered into the piperylene tower. With the method provided by the invention, piperylene product purity is improved, equipment investment is reduced, and operation cost is reduced.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for purifying dimethylchlorosilane by extraction and distillation
The invention discloses a method for purifying dimethylchlorosilane, which is to add an extractant to extract and distillate a low-boiling mixture produced in a process for producing methyl chlorosilane by a direct method to separate the dimethylchlorosilane, wherein extractant is an apolar aprotic solvent having a boiling point of no less than 70 DEG C. The extraction and distillation method adopted can effectively separate a hydrocarbon of 2-methyl-2-butylene having a boiling point similar to that of the dimethylchlorosilane, so the method for purifying the dimethylchlorosilane by extraction and distillation breaks a limit of the conventional distillation purification method that the maximum content of the dimethylchlorosilane is less than 88 percent, achieves a content of the dimethylchlorosilane in distillation of up to 95 percent, even 98 percent.
Owner:JIAXING UNITED CHEM +1
Method for preparing adiponitrile
InactiveCN101985431ACarboxylic acid nitrile preparationOrganic compound preparationReaction temperatureMethyl group
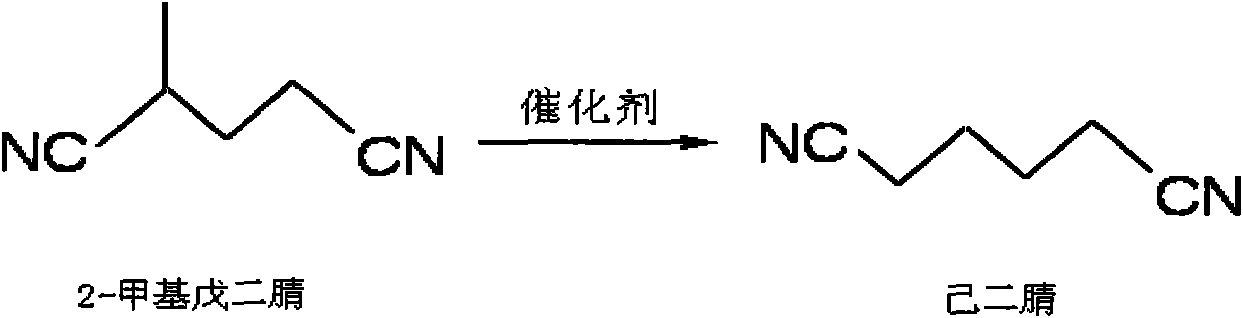
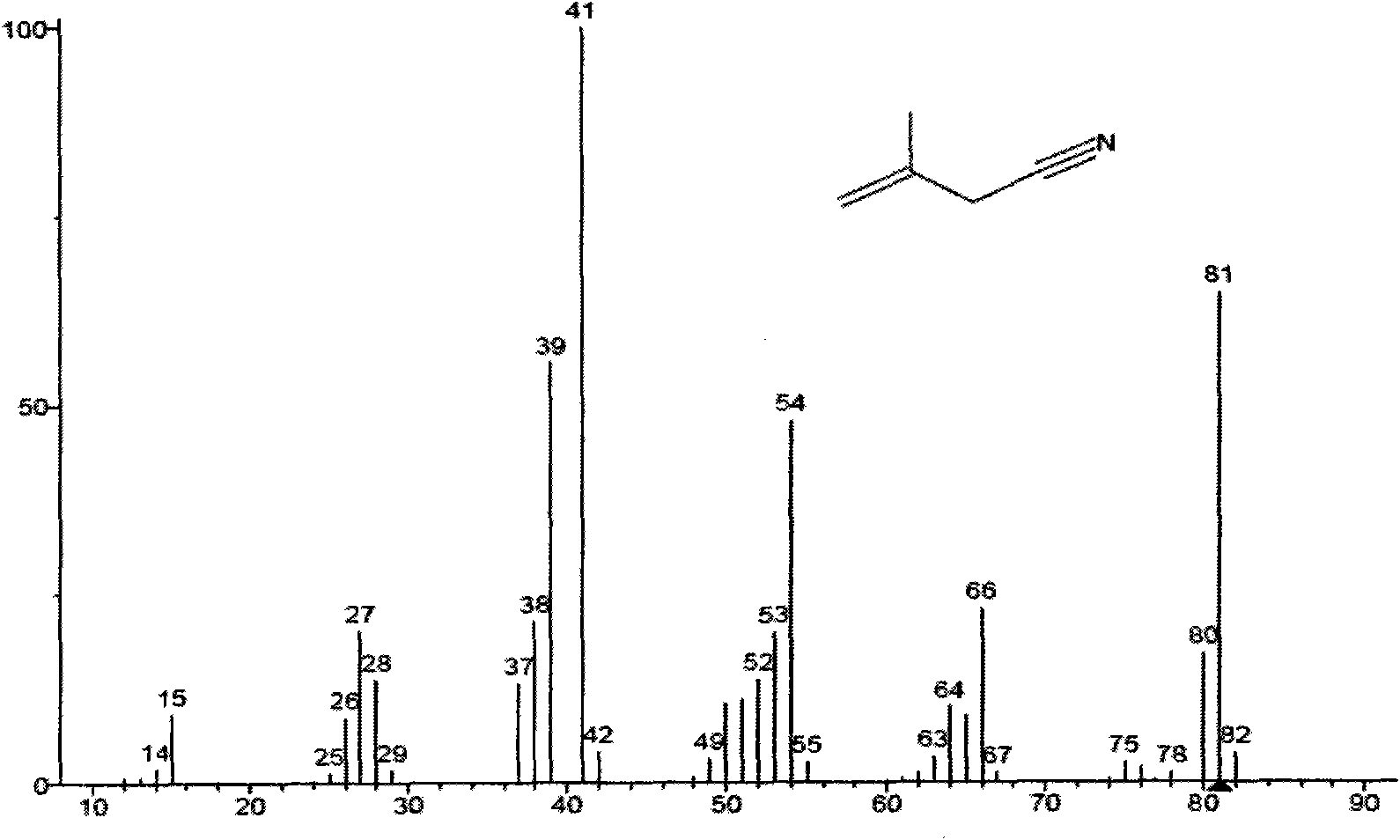
The invention discloses a method for preparing adiponitrile, relates to reaction for isomerizing branched chain adiponitrile into linear adiponitrile in the technical field of chemical industry, in particular to the method for preparing the adiponitrile. In the method, 2-methyl glutaronitrile is isomerized into the adiponitrile; and the 2-methyl glutaronitrile is independently isomerized or mixedwith other compounds and then the mixture is isomerized in an organic solvent in the presence of a transition metal organic compound serving as a catalyst, wherein the other compounds are 2-methyl-3-butene nitrile, 2-methyl-2-butene nitrile, 4-amylene nitrile, 3-amylene nitrile, 2-amylene nitrile, butadiene nitrile, the adiponitrile, and 2-ethyl butanedinitrile or valeronitrile; and the reaction temperature is between 10 and 250 DEG C. The 2-methyl glutaronitrile is one of main byproducts in the synthesis industry of the adiponitrile and has a small practical application range at present. By designing appropriate reaction conditions and selecting a high-efficiency catalyst, the reaction for isomerizing the 2-methyl glutaronitrile into the adiponitrile is realized and the 2-methyl glutaronitrile is isomerized into the linear adiponitrile, so that a new field is created for the industrial application of the 2-methyl glutaronitrile and the method certainly has profound effect on the conventional synthesis industry of the adiponitrile.
Owner:NANKAI UNIV
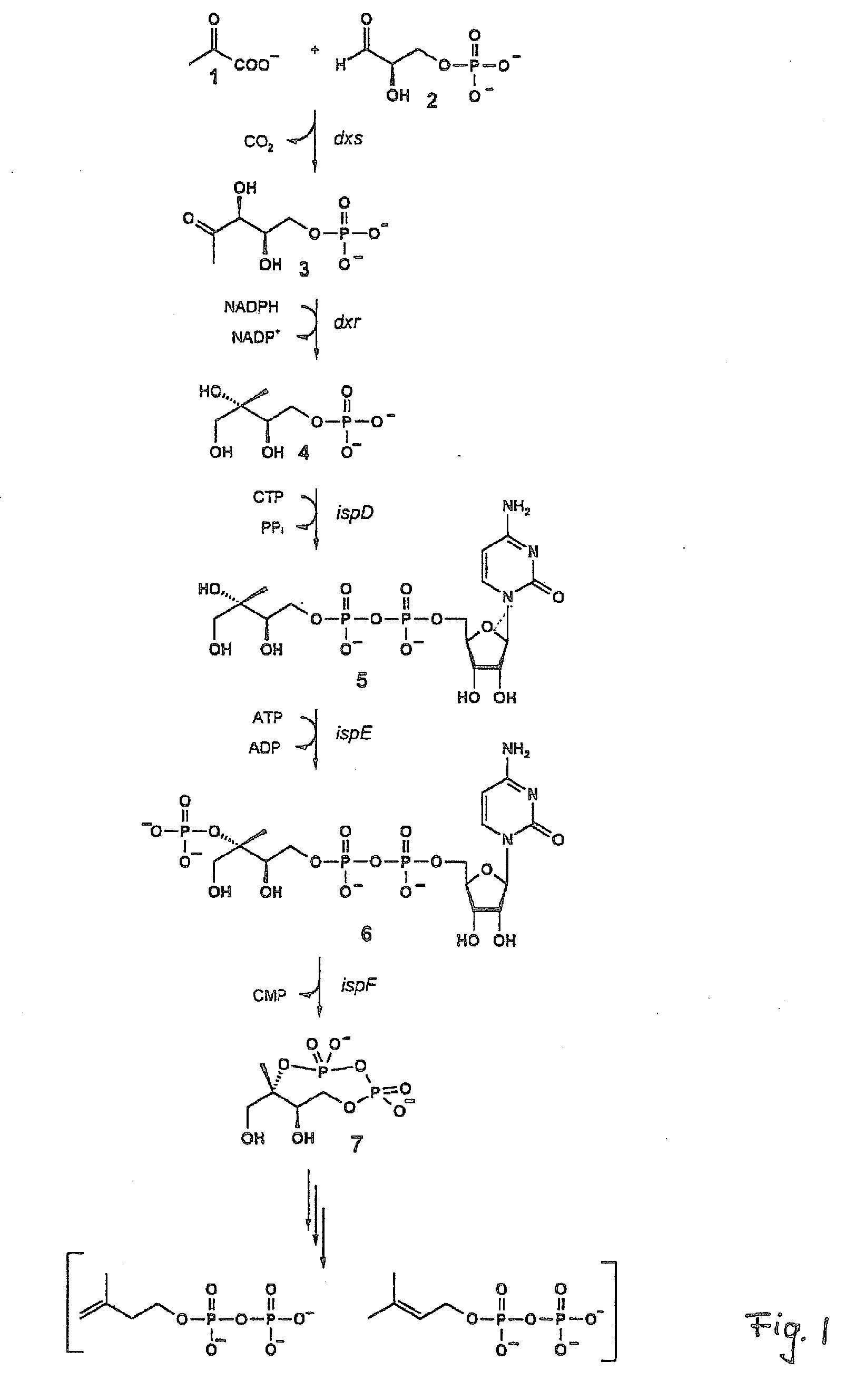
Intermediates and enzymes of the non-mevalonate isoprenoid pathway
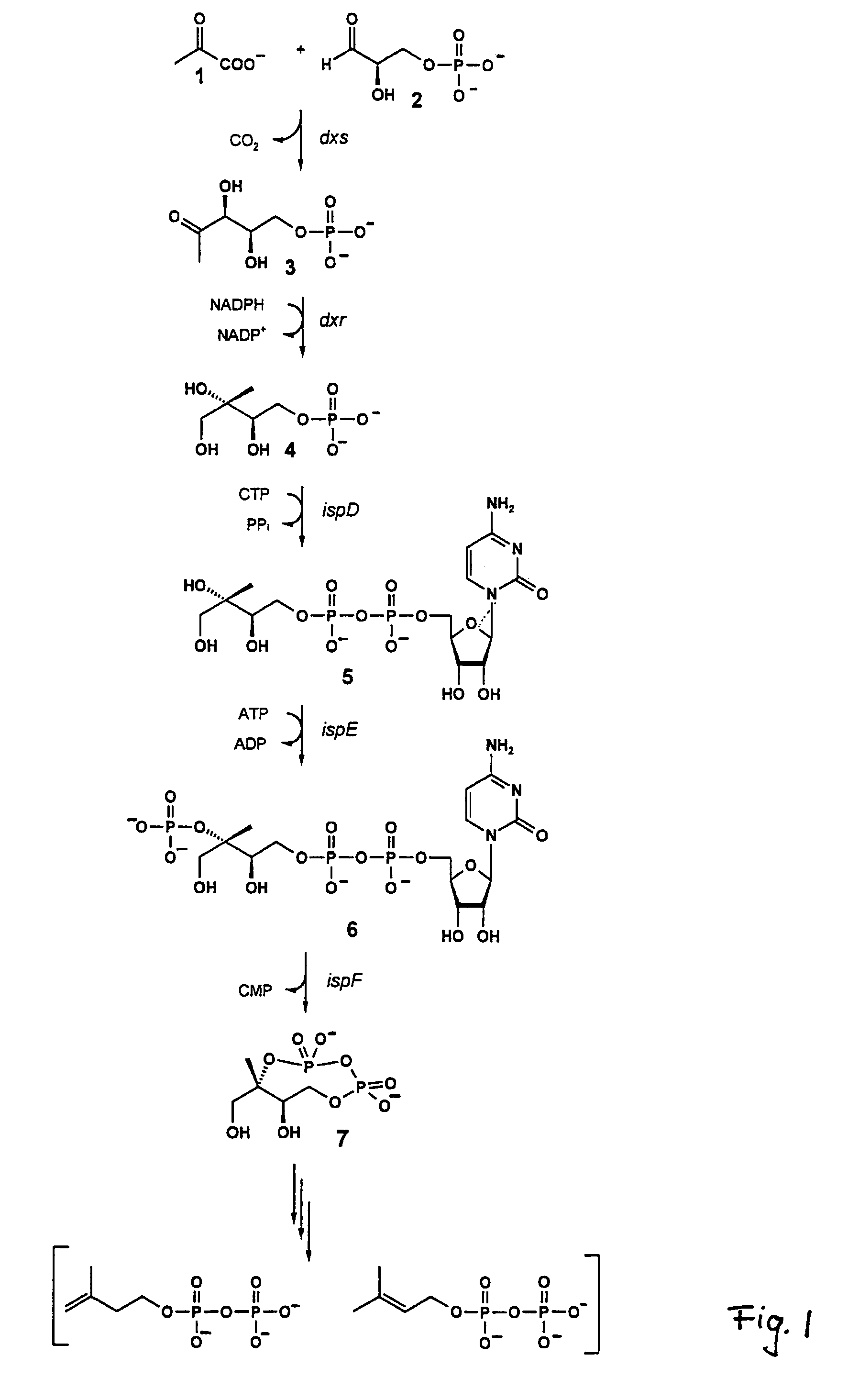
The invention provides a protein in a form that is functional for the enzymatic conversion of 2C-methyl-D-erythritol 2,4-cyclodiphosphate to 1-hydroxy-2-methyl-2-butenyl 4-diphosphate notably in its (E)-form of the non-mevalonate biosynthetic pathway to isoprenoids. The invention also provides a protein in a form that is functional for the enzymatic conversion of 1-hydroxy-2-methyl-2-butenyl 4-diphosphate, notably in its (E)-form, to isopentenyl diphosphate and / or dimethylallyl diphosphate. Further, screening methods for inhibitors of these proteins are provided. Further, 1-hydroxy-2-methyl-2-butenyl 4-diphosphate is provided and chemical and enzymatic methods of its preparation.
Owner:BACHER ADELBERT +1
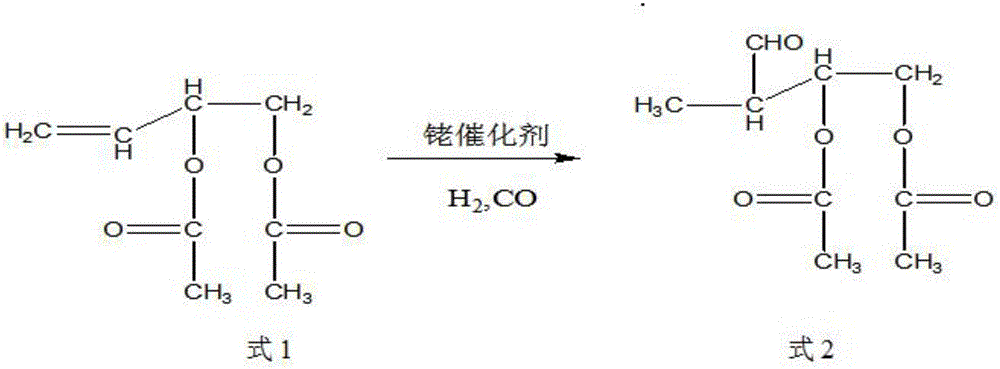
Synthetic method of trans-4-acetoxyl-2-methyl-2-butene-1-aldehyde
InactiveCN105968008AHigh selectivityLow reaction pressureOrganic compound preparationOrganic chemistry methodsFormylation reactionSolvent
The invention discloses a synthetic method of trans-4-acetoxyl-2-methyl-2-butene-1-aldehyde. The method comprises the steps that 3,4-diacetoxy-1-butene and synthetic gas are used as raw materials, a formylation reaction is carried out at the temperature of 85-100 DEG C and pressure of 85-100 kg under the effects of a rhodium catalyst and an additive, the synthetic gas is mixed gas of nitrogen and carbon monoxide, and 3-formoxyl-1,2-butanediol diethyl ester is prepared; the 3-formoxyl-1,2-butanediol diethyl ester is subjected to a decarboxylic reaction at the temperature of 85-120 DEG C through an acid catalyst, and the trans-4-acetoxyl-2-methyl-2-butene-1-aldehyde is obtained. By adding the additive in the formylation reaction and adjusting the mass ratio of the additive to rhodium in the rhodium catalyst, the formylation selectivity is greatly improved, and the formylation reaction pressure is lowered; no solvent is needed, the loss caused by solvent recycling can be lowered, the production cost and production difficulty are reduced, and the raw material utilization rate is improved.
Owner:FUJIAN FUERJIN BIOTECHNOLOGY CO LTD
Preparation method of 4-acetoxyl-2-methyl-2-butene-1-aldehyde
InactiveCN101817747AReduce pollutionHigh purityOrganic compound preparationCarboxylic acid esters preparationCatalytic oxidationSolvent
The invention discloses a preparation method of 4-acetoxyl-2-methyl-2-butene-1-aldehyde, comprising the step of: carrying out catalytic oxidation on 4-acetoxyl-2-butene-1-alcohol as a raw material and pure oxygen or air as an oxidizing agent, the series compound of a tetramethyl-piperidine oxide as a main catalyst and ferric nitrate as an assistant in a solvent under the condition of a certain temperature to obtain the 4-acetoxyl-2-methyl-2-butene-1-aldehyde. The preparation method has the characteristics of high selectivity, mild reaction condition and simple operation; and solid wastes can not be generated in the reaction process, and products are easy to refine.
Owner:ZHEJIANG MEDICINE CO LTD
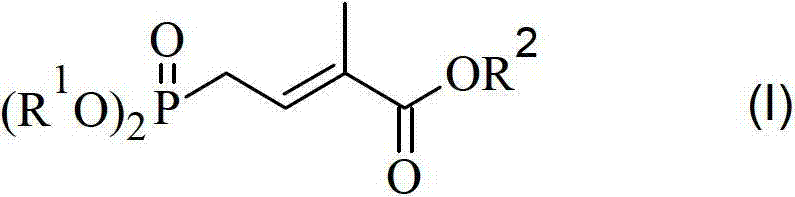
Method for preparing 4-bialkoxy-phosphono-2-methyl-2-butenoic acid alkyl ester
ActiveCN103113404AConvenient sourceReliable responseGroup 5/15 element organic compounds2 Butenoic Acids2-Methyl-2-butene
The invention discloses a method for preparing 4-bialkoxy-phosphono-2-methyl-2-butenoic acid alkyl ester. According to the method, a compound shown in a formula A and a compound shown in a formula B have reaction to generate a compound having a general formula I. The method has the advantages that raw materials are easily available sources, reaction is stable and reliable, reaction conditions are mild, and environment-friendliness effect is achieved, and the method is very suitable for industrial production.
Owner:广州巨元生化有限公司
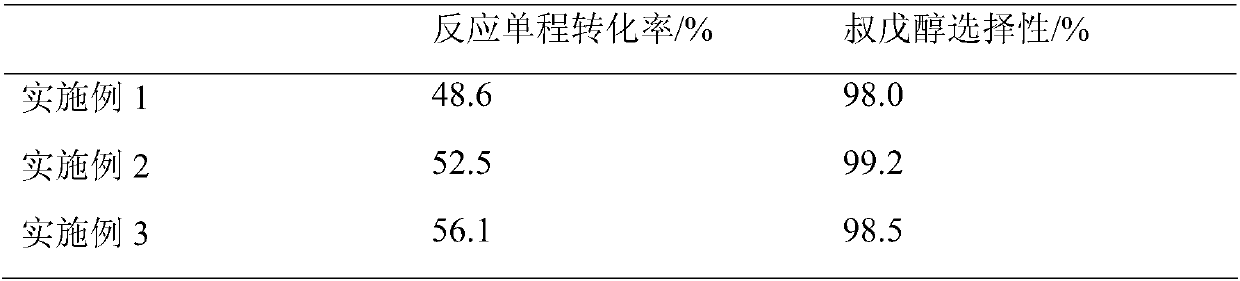
Method for preparation of tert-amyl alcohol by isoamylene hydration
InactiveCN108017508ARaise the ratioReduce the ratioPreparation by hydroxy group additionHydration reactionFixed bed
The invention discloses a method for preparation of tert-amyl alcohol by isoamylene hydration. The method specifically includes the steps of: mixing an isoamylene component rich in 2-methyl-1-butene and 2-methyl-2-butene, water and acetone, then loading the mixture into a fixed bed reactor holding a strong acidic cation exchange resin catalyst, and carrying out hydration reaction to obtain tert-amyl alcohol. The method provided by the invention uses acetone as the solvent, which is free of azeotropy with water and is easy to recover; the solvent ratio is low, the energy consumption of reactionproduct post-treatment is saved, and the production operation cost is also saved.
Owner:CHINA PETROLEUM & CHEM CORP +1
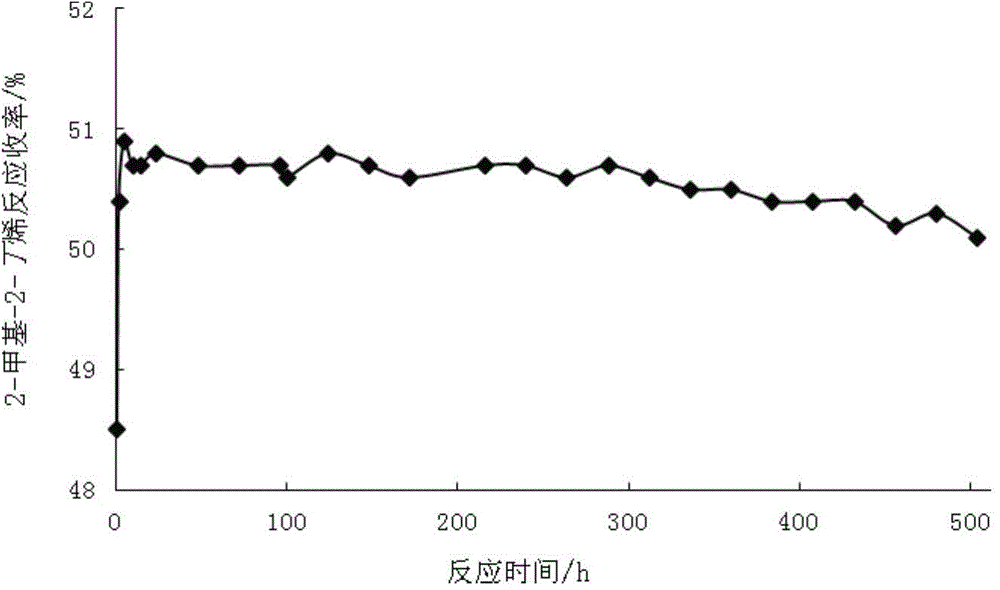
A preparing method of 2-methyl-2-butene
InactiveCN104557379AHigh reaction yieldExcellent anti-coking performanceHydrocarbon by isomerisation2-methylbutaneIsomerization
The invention relates to a preparing method of 2-methyl-2-butene. The method comprises following steps of: 1) feeding a raffinate C5 raw material into a first-section fixed-bed reactor, with the middle of the first-section fixed-bed reactor being filled with a ZSM-5 sodium type aluminosilicate crystal molecular sieve, and allowing n-pentene in the raffinate C5 to be isomerized into isopentene; 2) feeding the reaction materials obtained in the step 1) into a second-section fixed bed isomerization reactor and isomerizing 2-methyl-1-butene into 2-methyl-2-butene; 3) feeding the reaction materials in the step 2) into a rectifying tower for rectification, discharging 3-methyl-1-butene, n-pentane, isopentane, a small amount of unreacted 1-pentene, 2-methyl-1-butene, and other components from the tower top, and obtaining unreacted 2-pentene, 2-methyl-2-butene and a small amount of n-pentane and heavy components from the tower bottom; 4) feeding the tower bottom material obtained in the step 3) into a rectifying tower for continuous rectification, obtaining n-pentane and 2-pentene from the tower top, and obtaining 2-methyl-2-butene and a small amount of heavy components from the tower bottom; and 5) feeding the materials discharged from the tower top in the step 4) to a feeding port of a first-section isomerization reactor, mixing with a fresh raffinate C5 raw material, and feeding the mixture to the first-section isomerization reactor. Compared with the prior art, the method has advantages of high reaction yields, good anti-coking performance, long catalyst service lifetime, and the like.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing isoamylene by using tertiary amyl methyl ether
InactiveCN102040452ASlow reaction rateReduce acid strengthHydrocarbon by isomerisationIsomerizationBiopolymer
The invention relates to a method for preparing isoamylene by using tertiary amyl methyl ether, comprising: etherification reaction is carried out on the gas-phase tertiary amyl methyl ether by a catalyst bed layer; isomerization reaction is carried out on liquid-phase methanol-contained products in etherification reaction by means of the catalyst bed layer, so that 2-methyl-1-butylene in the etherification products is transformed into 2-methyl-2-butylene,wherein the quality air speed is 1-30hr<-1>, the reaction temperature is 20-47 DEG C, the reaction pressure is 0.3-1.0MPa, a catalyst is sulfonic cation exchange resin, and the quality exchange capacity is 3-5.5mmol / g; the methanol is removed from the products in the isomerization reaction, thus the isoamylene is obtained. By utilizing the method provided by the invention, the dimerization reaction during the isomerization reaction is effectively inhibited, thus the content of biopolymers in the product can be reduced to below 1%; only the sequence of the technical steps in the prior art is changed, other matters are not introduced into a reaction system, extra load can not be added for purifying or refining the product, and the defects in the prior art are effectively overcome.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method of preparing zeitin compound by applying ionic liquid
InactiveCN103524585AReduce usageShorten the timeSugar derivativesSugar derivatives preparationNitrogen2-Methyl-2-butene
The invention relates to a method of preparing a zeitin compound by applying ionic liquid. According to the method, isoprene is used as a starting material to carry out a one-pot reaction in the ionic liquid, i.e., (E)-4-(phthalimide)-2-methyl-2-butenyl acetate is obtained through bromination, selective nitrogen substitution as well as acetoxy substitution reaction, and then (E)-4-hydroxy-3-methyl-2-butenyl amine is obtained through hydrazinolysis and the zeitin compound is conveniently obtained through continuous reaction. The method disclosed by the invention has the advantages of being simple in synthesis route, green and environment-friendly, easy for industrialization, low in product cost as well as high in product purity at the same time, and the like.
Owner:BEIJING HARMONY CHEM TECH
Method for recycling tetrahydrofuran in tetrahydrofuran-methanol-water by single-tower extractive distillation
ActiveCN105968073AReduce energy consumptionReduce separation costsOrganic compound preparationChemical industryMethanol waterExtractive distillation
The invention provides a method for recycling tetrahydrofuran in tetrahydrofuran-methanol-water by single-tower extractive distillation, aiming at a current situation that two groups of azeotropes (namely tetrahydrofuran-methanol and tetrahydrofuran-water) in a tetrahydrofuran-methanol-water ternary mixture are difficult to separate. The method takes 2-methyl-2-butene as an extracting agent and realizes a target of recycling the high-purity tetrahydrofuran from the ternary mixture by utilizing the characteristic that the 2-methyl-2-butene, methanol and water form the lowest azeotrope. By adopting single-tower extractive distillation operation, compared with a traditional separation method, the energy consumption is greatly reduced and equipment cost is reduced. The extracting agent 2-methyl-2-butene is not dissolved in the water, so that high-purity recycling is realized; the extracting agent can be recycled to an extractive distillation tower and the cost of the raw materials is reduced.
Owner:QINGDAO UNIV OF SCI & TECH
A method of preparing high-purity 1-pentene
ActiveCN104557409AAdd recovery unitEasy to separateChemical modification purification/separationAlkaneButene
A method of preparing high-purity 1-pentene is disclosed. The method includes steps of: 1) rectifying a raw material that is raffinate C5 after extraction and separation of diolefine and isopentene so as to separate and remove heavy components, obtaining an enriched 1-pentene fraction from the tower top, and discharging heavy components from the tower bottom; 2) subjecting the enriched 1-pentene fraction obtained from the tower top in the step 1) to precision rectification so as to remove light components, obtaining C5 alkanes and other light components from the tower top, and obtaining a 1-pentene crude product from the tower bottom; 3) subjecting the 1-pentene crude product obtained in the step 2) to isomerization through a sulfo-group cation exchange resin fixed catalytic bed to convert 2-methyl-1-butene that is difficult to separate in the crude product into 2-methyl-2-butene that is easy to separate; and 4) feeding isomerization products obtained in the step 3) to a rectification tower, and purifying to obtain the 1-pentene having purity higher than 98%. By improving process steps, the method simplifies the process, reduces the production cost, and improves the economical efficiency of the process of preparing the high-purity 1-pentene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Separating a solvent from a nickel catalyst by distillation
ActiveCN105531257AOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst regeneration/reactivationNickel catalystIsomerization
A solvent is at least partially separated from a catalyst. The catalyst comprises nickel and a bidentate phosphorus-containing ligand. The method for separation involves distilling a catalyst solution. The ratio of 2-pentenenitrile to 3-pentenenitrile in distillation column bottoms is controlled to reduce the amount of 3-pentenenitrile which is isomerized to form 2-methyl-3-butenenitrile. Isomerization of 3-pentenenitrile to 2-methyl-3-butenenitrile, and subsequent isomerization of 2-methyl-3-butenenitrile to 2-methyl-2-butenenitrile, and / or hydrocyanation of 2-methyl-3-butenenitrile to methylglutaronitrile represents a loss in adiponitrile yield in a process for making adiponitrile.
Owner:INVISTA TEXTILES (U K) LTD
Renewable olefins from a mixture of acetic acid and propionic acid
ActiveUS9212106B2Improve stabilityImprove impact resistanceThermal non-catalytic crackingHydrocarbon by isomerisationButeneAcetic acid
A process is described for making a product mixture including isobutene, propylene, 1-butene, 2-butene, 2-methyl-1-butene and 2-methyl-2-butene from a mixture of acetic acid and propionic and through reaction in the presence of a source of hydrogen and of a mixed oxide catalyst, for example, a ZnxZryOz mixed oxide catalyst. A variety of commercially valuable products may be made in turn from the various C3, C4 and C5 constituents of the product mixture.
Owner:WASHINGTON STATE UNIVERSITY +1
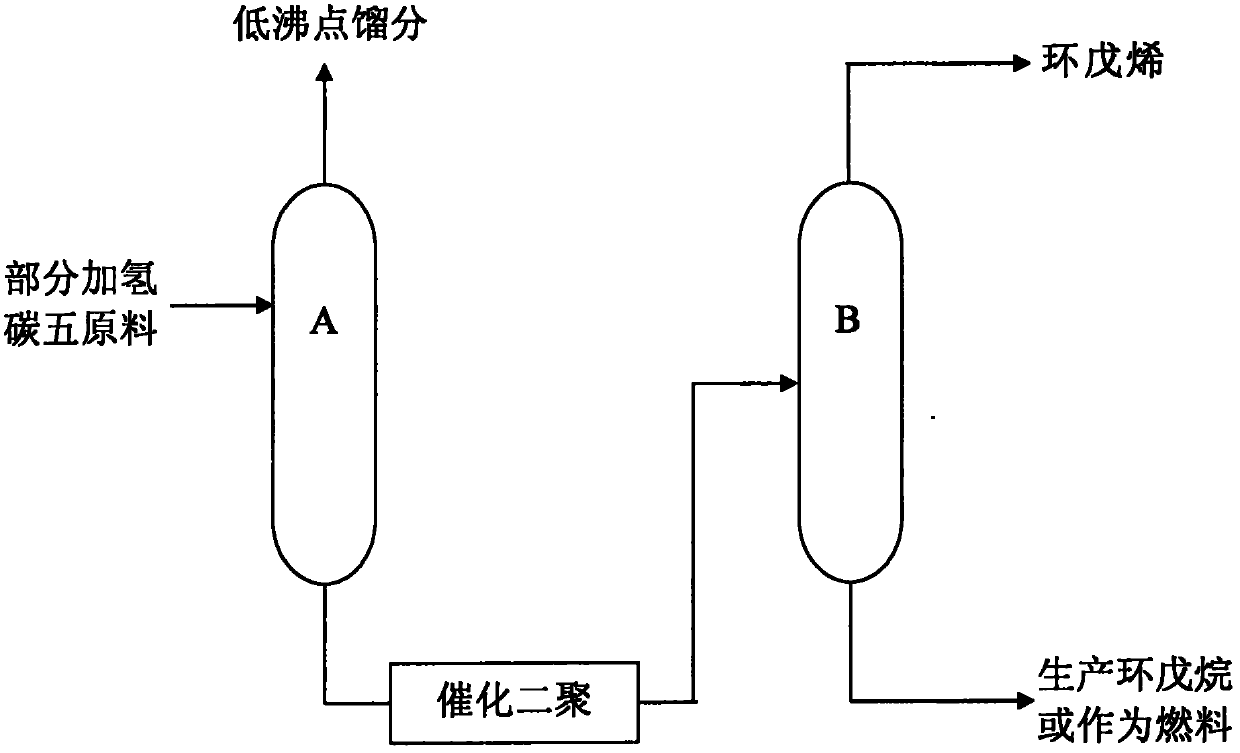
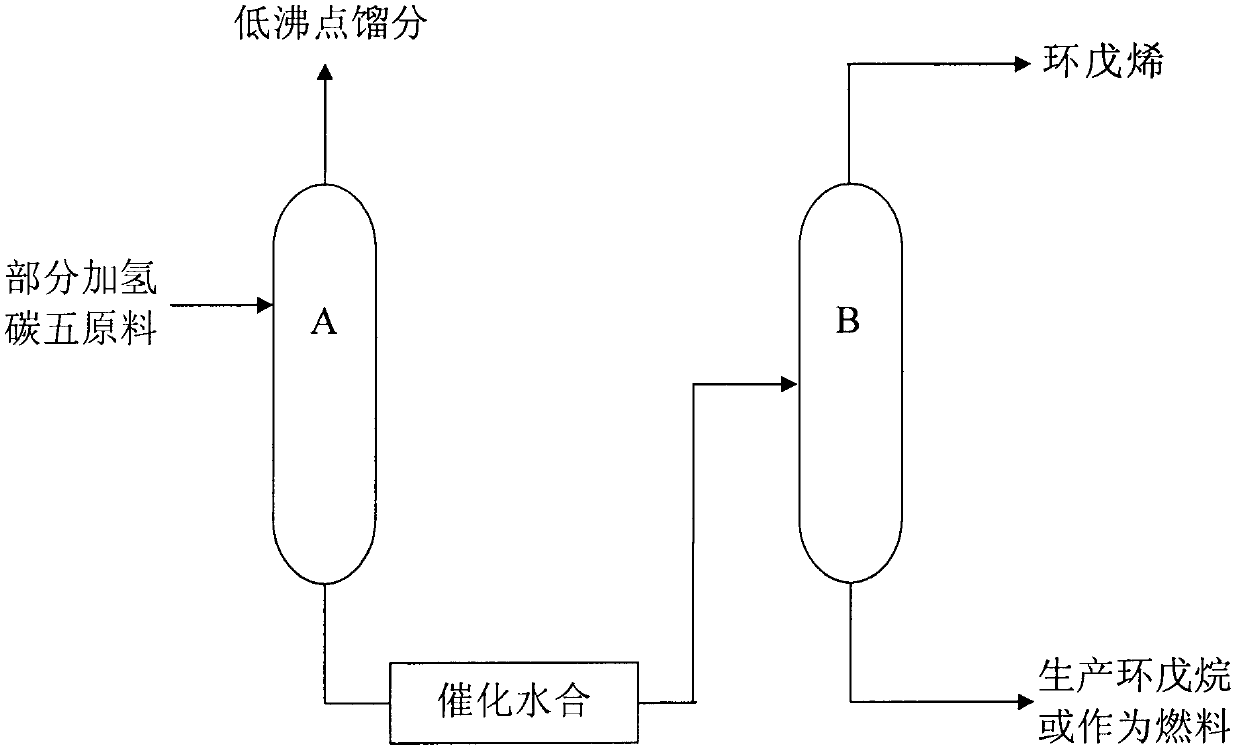
Production method of high purity cyclopentene
InactiveCN103304361AEasy to separateReduce energy consumptionDistillation purification/separationHydrocarbonsCyclopenteneButene
The invention relates to the technical field of production of chemical products, and in particular relates to a production method of high purity cyclopentene. The method comprises the following steps of: feeding part of a hydrogenated c5 fraction to a rectifying tower A; separating a coarse isoamylene fraction from the top; obtaining a tower bottom liquid comprising cyclopentene and cyclopentane fractions from the kettle; feeding the tower bottom liquid after dimerization reaction to a rectifying tower B for rectification; and extracting cyclopentene from the top. The method provided by the invention has the advantages that when the part of hydrogenated c5 fraction is separated to produce cyclopentene, 2-methyl-2-butene which is very approximate to cyclopentene in boiling point is converted into a heavy component with higher boiling point through the dimerization reaction on a catalyst bed layer, so that cyclopentene is easily separated from 2-methyl-2-butene, so that not only is the energy consumption remarkably reduced, but also cyclopentene with purity over 99% can be produced.
Owner:上海博润石化科技发展有限公司 +1
Renewable olefins from a mixture of acetic acid and propionic acid
ActiveUS20140128650A1Improve impact resistanceImprove stabilityThermal non-catalytic crackingHydrocarbon by isomerisationButeneAcetic acid
A process is described for making a product mixture including isobutene, propylene, 1-butene, 2-butene 2-methyl-1-butene and 2-methyl-2-butene from a mixture of acetic acid and propionic and through reaction in the presence of a source of hydrogen and of a mixed oxide catalyst, for example, a ZnxZryOz mixed oxide catalyst. A variety of commercially valuable products may be made in turn from the various C3, C4 and C3 constituents of the product mixture
Owner:WASHINGTON STATE UNIVERSITY +1
Preparation method of high purity cyclopentene
InactiveCN103304362AAchieve the purpose of separationReduce energy consumptionDistillation purification/separationChemical modification purification/separationHydration reactionButene
The invention relates to the technical field of production of chemical products, and in particular relates to a preparation method of high purity cyclopentene. The method comprises the following steps of: feeding part of a hydrogenated c5 fraction to a rectifying tower A; separating a coarse isoamylene fraction from the top; obtaining a tower bottom liquid comprising cyclopentene and cyclopentane fractions from the kettle; feeding the tower bottom liquid after hydration reaction to a rectifying tower B for rectification; and extracting cyclopentene from the top. The method provided by the invention has the advantages that when the part of hydrogenated c5 fraction is separated to produce cyclopentene, 2-methyl-2-butene which is very approximate to cyclopentene in boiling point is converted into a heavy component with higher boiling point through the dimerization reaction on a catalyst bed layer, so that cyclopentene is easily separated from 2-methyl-2-butene, so that not only is the energy consumption remarkably reduced, but also cyclopentene with purity over 99% can be produced.
Owner:上海博润石化科技发展有限公司 +1
Method for improving content of 2-methyl-2-butylene
ActiveCN106588550AImprove quality ratioThe isomerization reaction goes wellHydrocarbon by isomerisationCatalystsTert-Amyl methyl etherIsomerization
The invention discloses a method for improving the content of 2-methyl-2-butylene and particularly relates to a method for improving the content of 2-methyl-2-butylene in isopentene by taking tert-amyl methyl ether as a raw material and adopting methods for ether reaction, isomerization reaction and purification by rectification. Through the technical processes, the mass ratio of 2-methyl-2-butylene to 2-methyl-1-butylene in isopentene can be improved to (8-14):1, so that the quality requirements for subsequent processing are met.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparing method of tert-amyl acetate
ActiveCN102126946ATo achieve the effect of suppressing side effectsReduce usageOrganic compound preparationCarboxylic acid esters preparationAmyl acetateMethyl group
The present invention relates to a method for preparing tert-amyl acetate through the reaction between an alkene compound with 5-6 carbon atoms and an acetic acid. More particularly, a low boiling point alkene mixture of fluidized catalytically cracked naphtha which is obtained through a naphtha cracking technique and contains the alkene with five to six carbon atoms in each module as the main component is used. In the mixture, only 2-methyl-1-butylene and 2-methyl-butylene are selected from the mixture for reacting with the acetic acid, and the purpose is obtaining high-purity tert-amyl acetate. Furthermore, the invention relates to a method for easily separating the alkene component which has high possibility of incomplete combustion in the low boiling point catalytically cracked naphtha.
Owner:SK INNOVATION CO LTD +1
Methanol fuel composite additive favorable for cold boot of compressing ignition engine
ActiveCN106520230AChange low temperature volatility performanceImprove low temperature fluidityLiquid carbonaceous fuelsCooling effectEngineering
The invention provides a methanol fuel composite additive favorable for the cold boot of a compressing ignition engine. The methanol fuel composite additive is prepared from the following ingredients in percentage by mass: 34-45% of cosolvent, 0.5-1% of antioxygen, 0.5-1% of corrosion inhibitor for metal and the balance of a main reagent, wherein the main reagent is formed by blending acetone, methyl acetate, cyclopentane, ethylene glycol diethyl ether, 1,1-dimethoxyethane, bromoethane and 2-methyl-2-butene, and the cosolvent is formed by blending isobutyl alcohol, isoamyl alcohol, octanol and tert-butyl acetate. According to the methanol fuel composite additive disclosed by the invention, the low temperature volatilization performance of methanol is changed, the gasification degree and the evaporation rate of the methanol are increased when the methanol is burnt, the low temperature fluidity of the methanol is improved, influence on engine operation performance by a cooling effect generated by the high gasification latent heat of the methanol is avoided, and an engine can be successfully booted at the temperature of -40DEG C.
Owner:ENERGY & ENVIRONMENT RES INST OF HEILONGJIANG PROVINCE +1
Method for preparing isoamylene by taking tertiary amyl methyl ether as raw material
ActiveCN101723777BIncreased 2-methyl-2-butene contentInhibition of dimerizationHydrocarbon by isomerisationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationGas phase
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Dichloromethane purification method and process for producing difluoromethane using the same
InactiveUS20160060193A1Simple and efficient mannerHigh yieldPreparation by halogen halide additionPreparation by halogen replacementPurification methodsHydroquinone Compound
To provide a dichloromethane purification method which can reduce the amount of stabilizers present in dichloromethane and is feasible in industry by a simple operation, a dichloromethane purification method includes bringing dichloromethane containing at least one stabilizer selected from the group consisting of 2-methyl-2-butene, hydroquinone and resorcinol into contact in a liquid phase state with a zeolite having an average pore size of 3 to 11 Å and thereby reducing the amount of the stabilizer.
Owner:SHOWA DENKO KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com