Method of preparing zeitin compound by applying ionic liquid
An ionic liquid and compound technology, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of poor N substitution reaction selectivity, long bromine dripping process, and difficulty in realizing industrialization. High selectivity, reducing the time for dripping bromine, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the preparation of ionic liquid
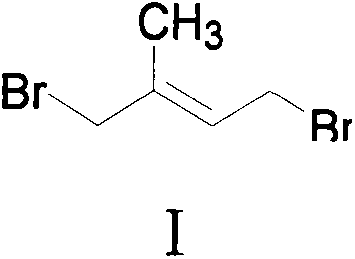
[0060] Ionic liquid [Bmim]Br 3 (i.e. 1-butyl-3-methylimidazole tribromide) preparation: 821g (10.0mol) of 1-methylimidazole was dissolved in 5L toluene, and 1437g (10.5mol) of 1-methylimidazole was added dropwise while stirring. Bromobutane, after the dropwise addition, heat to reflux for 24 hours, cool and stand still, separate the toluene, add 3L of ethyl acetate, and ultrasonicate while stirring, a large amount of white solid precipitates, filter, and wash the solid with ethyl acetate for 2 to 3 times , dried in vacuo to obtain ionic liquid [Bmim]Br (namely 1-butyl-3-methylimidazole bromide). Add 1440g (9.0mol) of bromine dropwise to [Bmim]Br while stirring, the mixture slowly turns from white to orange-yellow liquid, after the dropwise addition, continue to stir for 2 hours, add 1L of ethyl acetate to wash, then vacuum dry . Obtain orange-yellow ionic liquid [Bmim]Br 3 .
[0061] Ionic liquid [Emim]Br 3 (i.e. 1-e...
Embodiment 2
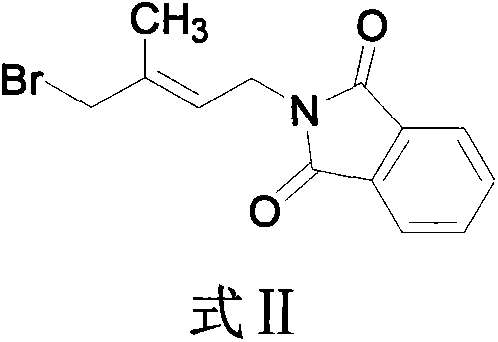
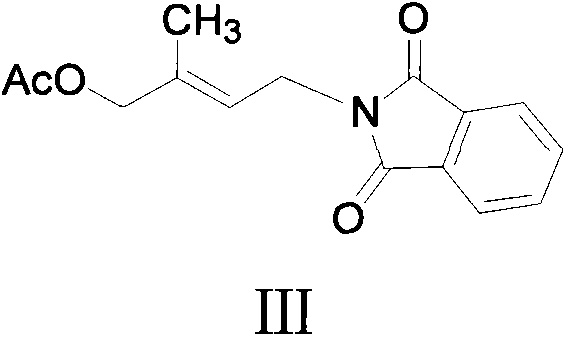
[0062] Example 2: One-pot preparation of (E)-4-(phthalimide)-2-methyl-2-butenyl acetate (i.e. compound III) with ionic liquid:
[0063]
[0064] (A) Add isoprene to the reaction kettle, control the temperature at -5°C to 25°C, add [Emim]Br dropwise 3 or [Bmim]Br 3 After the dropwise addition, continue to stir for 1 hour, distill off the excess isoprene, and the main product obtained is compound I, which is not treated, and continues to the next step of reaction.
[0065] (B) add dimethylformamide (DMF) in reactor, K 2 CO 3 , temperature control 0 ℃ ~ 25 ℃, while stirring, dropwise add the DMF solution of phthalimide; stir for about 6 hours, that is, the reaction ends when the phthalimide reaction is completed, and the main product obtained It is compound II, no treatment, continue to the next step reaction.
[0066] (C) Add sodium acetate (NaOAc) to the reaction solution in the previous step, heat to 80°C to 110°C, stir for 2 hours, finish the reaction with raw material...
Embodiment 3
[0067] Embodiment 3: the recovery of ionic liquid
[0068] Recovery of ionic liquid: in step (C) in embodiment 2, after toluene extracts organic compound, the main substance that obtains is ionic liquid [Emim]Br or [Bmim]Br, in this ionic liquid [Emim]Br or Add ethyl acetate to [Bmim]Br to wash for 2 to 3 times, and vacuum dry; then add bromine dropwise to [Emim]Br or [Bmim]Br to obtain [Emim]Br 3 or [Bmim]Br 3 , for repeated use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com