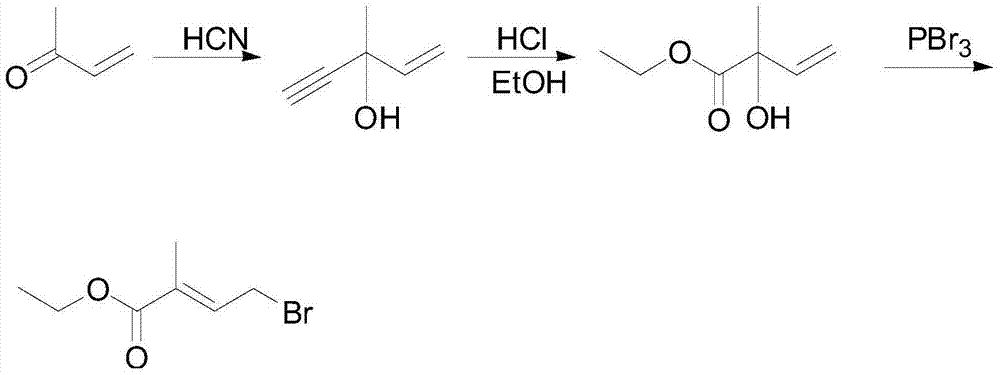
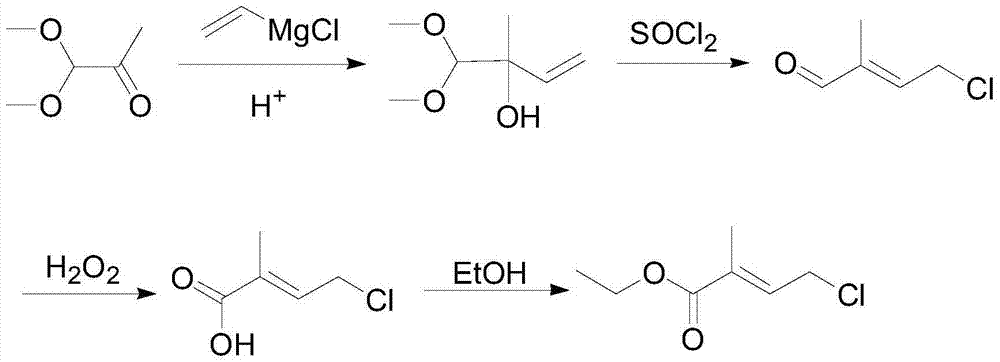
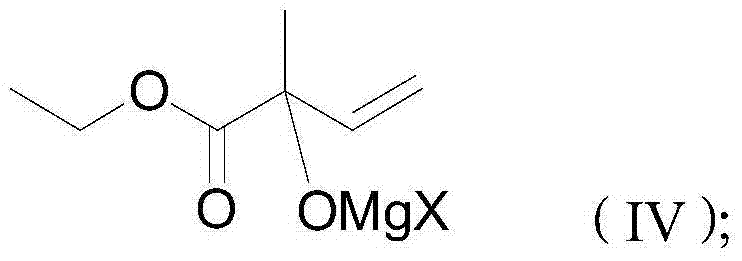4-halogenated-2-methyl-2-ethyl crotonate preparing method
A technology of ethyl crotonate and ethyl pyruvate, which is applied in the field of chemical intermediate synthesis, can solve the problems of low total reaction yield and long process route, and achieves good reaction selectivity, simple overall process and no equipment requirements. harsh effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A. Preparation of Hydroxyl Compound (V)
[0033] Add 23.43g of ethyl pyruvate (99% purity) and 80ml of toluene into a 250ml four-neck flask, stir to cool down to -10°C, and start to drop 91ml of vinylmagnesium chloride Grignard reagent (content: 2.2mol / L, commercially available product, the solvent is tetrahydrofuran), the dripping is completed in about 60 minutes, and the heat preservation reaction is 1 hour. After the heat preservation is completed, the resulting product is a condensate.
[0034] The condensate was slowly added dropwise to 150ml of dilute sulfuric acid (10% concentration by mass), and the reaction temperature was kept within 50°C during the dropping process, and the reaction was continued for 10 minutes after the dropping. The layers were separated, the upper organic phase was separated, and the lower aqueous phase was extracted twice with toluene, each time the amount of toluene was 30ml. Combine and extract toluene to the organic phase, neutralize...
Embodiment 2
[0038] A. Preparation of Hydroxyl Compound (V)
[0039] Add 23.43g of ethyl pyruvate (purity: 99%) and 80ml of toluene into a 250ml four-neck flask, stir and cool down to -10°C, start to drop 87ml of vinylmagnesium bromide Grignard reagent (content: 2.3mol / L, Commercially available products, the solvent is tetrahydrofuran), the dripping is completed in about 60 minutes, and the reaction is kept for 1 hour. After the heat preservation is completed, the resulting product is a condensate. The condensate was slowly added dropwise to 150ml of dilute sulfuric acid (10% concentration by mass), and the reaction temperature was kept within 50°C during the dropping process, and the reaction was continued for 10 minutes after the dropping. The layers were separated, the upper organic phase was separated, and the lower aqueous phase was extracted twice with toluene, each time the amount of toluene was 30ml. Combine and extract toluene to the organic phase, neutralize with 100ml saturate...
Embodiment 3
[0043] A. Hydroxy compound (V) is prepared in the same way as in Example 1
[0044] B. Preparation of Bromocarbapentamate (I)
[0045] Add 22.85g of hydroxyl compound (V) (purity: 94.5%) and 60ml of dichloromethane into a 250ml four-neck flask, stir and cool the reaction mixture to 0°C, start to drop 32.0g of hydrobromic acid (mass percentage concentration: 57%) , After about 30 minutes of dripping, keep warm for 20 minutes. The layers were separated, the organic phase was separated, and the aqueous phase was extracted with 20ml of dichloromethane and combined into the organic phase. The organic phase was neutralized with 80ml of saturated sodium bicarbonate and then washed once with 80ml of saturated sodium chloride. After washing, dichloromethane was recovered to obtain 28.7g of a concentrate, which was compared and analyzed by commercially available standard bromocarbon pentaester (I) GC , the purity is 91.8%, and the yield is 84.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com