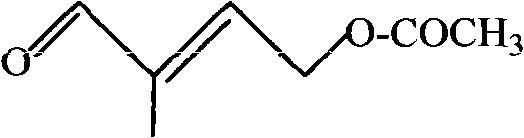
Preparation method of 4-acetoxyl-2-methyl-2-butene-1-aldehyde
An acetoxy and methyl technology, which is applied in the field of preparation of 4-acetoxy-2-methyl-2-butene-1-aldehyde, can solve the problems of environmental pollution, poor selectivity, complicated post-processing and the like, Achieve the effect of high purity, low environmental pollution and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
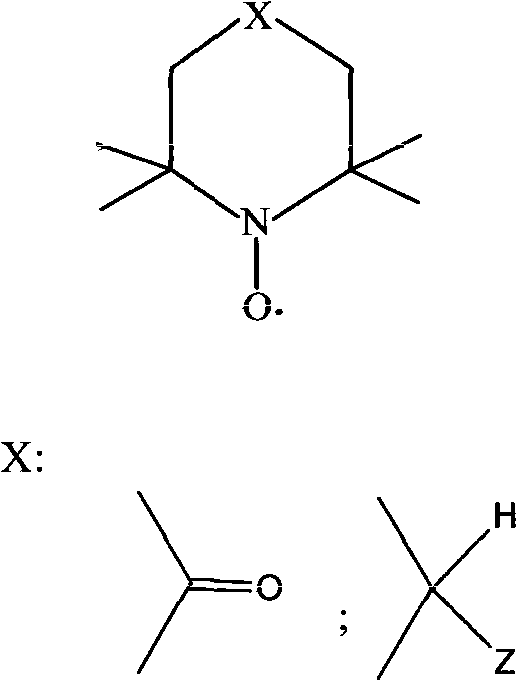
[0023] In a 100ml three-necked round-bottom flask equipped with magnetic stirring, add 14.40g (0.10mol) of 4-acetoxy-2-methyl-2-buten-1-ol and 20ml of benzotrifluoride as a solvent. Stir for 3-5min, then add THMPO 0.86g (5mmol) and Fe(NO 3 ) 3 9H 2 O 2.02g (5mmol), and slowly into the pure oxygen. Samples were taken after 3 hours of reaction, and GC analysis showed that the conversion rate of 4-acetoxy-2-methyl-2-buten-1-ol was 99.3%.
Embodiment 2
[0025] In a 100ml three-necked round-bottomed flask equipped with magnetic stirring, add 14.40g (0.10mol) of 4-acetoxy-2-methyl-2-buten-1-ol and 20ml of benzotrifluoride as a solvent. Stir for 3-5min, then add 4-O-TEMPO 0.52g (3mmol) and Fe(NO 3 ) 3 9H 2 O 1.21g (3mmol), and slowly into the pure oxygen. Samples were taken after 6 hours of reaction, and GC analysis showed that the conversion rate of 4-acetoxy-2-methyl-2-buten-1-ol was 99.6%.
Embodiment 3
[0027] In a 100ml three-necked round-bottomed flask equipped with magnetic stirring, add 14.40g (0.10mol) of 4-acetoxy-2-methyl-2-buten-1-ol and 20ml of benzotrifluoride as a solvent. Stir for 3-5min, then add 4-OCH in sequence 3 -TEMPO 0.86g (10mmol) and anhydrous Fe (NO 3 ) 3 2.02g (10mmol), and slowly into the pure oxygen. Samples were taken after 5 hours of reaction, and GC analysis showed that the conversion rate of 4-acetoxy-2-methyl-2-buten-1-ol was 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com