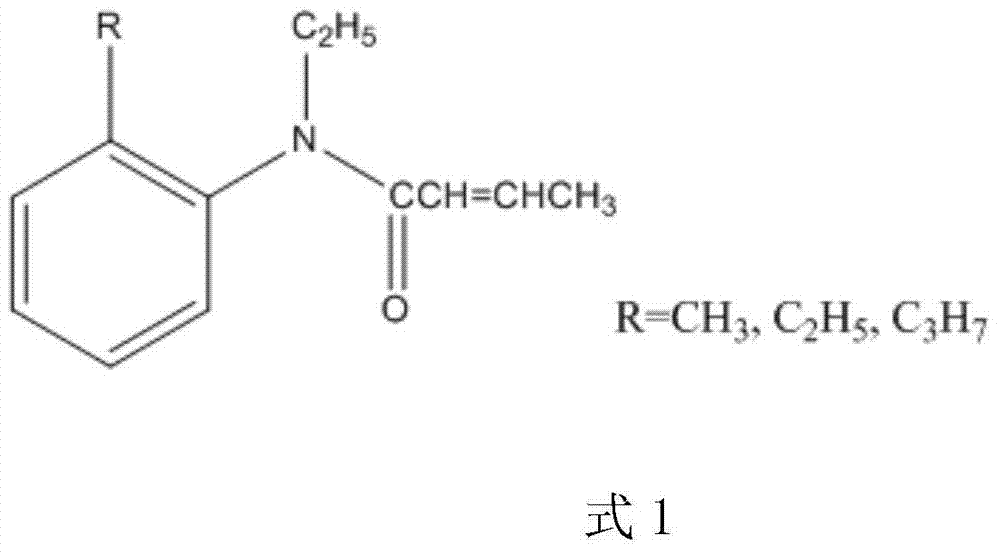
Synthesis method for trans N-ethyl-N-(2'-alkyl phenyl)-2-butenamide
A synthesis method and technology of alkylaniline, which are applied in the field of medicinal chemistry synthesis, can solve the problems that the reaction is not easy to operate, cannot obtain satisfactory purity and yield, difficult trans structure and the like, and achieve high product yield, easy operation and simple method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
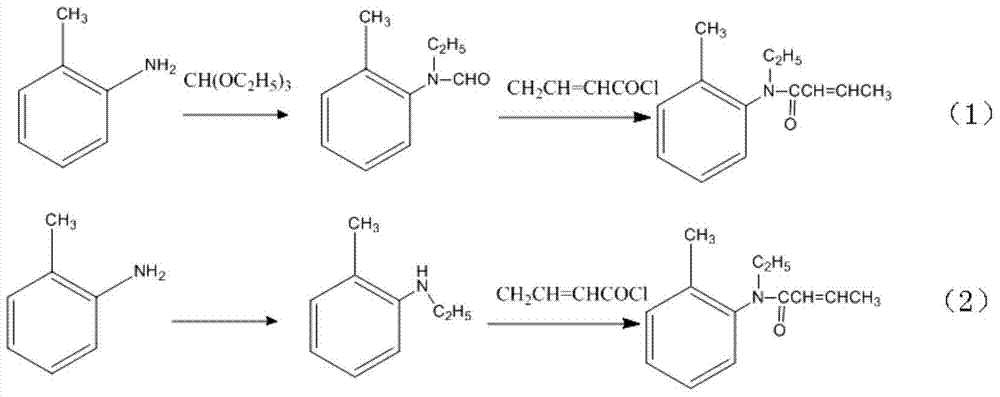
[0025] Put 20 g of trans-2-butenoic acid (content 99.1%, 0.23 mol) into a flask, add 20 g of petroleum ether, cool to 0-5° C. with ice water, and add 30 g (0.25 mol) of thionyl chloride dropwise. After the dropwise addition, stir at 20-30°C for 3 hours to obtain a 2-butenoyl chloride reaction solution for use.
[0026] Place 28 grams (0.21mol) of N-ethyl-2-methylaniline in another flask, add 20 grams of petroleum ether, then add 133g of 30% aqueous sodium hydroxide solution, keep at 10-20°C, and add dropwise under careful stirring The above 2-butenoyl chloride reaction solution was added dropwise within 30 minutes. Then react at 60-70°C, follow the reaction process in the gas phase, until the reactant N-ethyl-2-methylaniline basically reacts completely, about 6 hours.
[0027] After the reaction was completed, cool, separate the organic layer and wash with water, distill the petroleum ether off, distill the residual liquid under reduced pressure, collect the fraction at 102-1...
Embodiment 2
[0029] Put 4 g of trans-2-butenoic acid (content 99.1%, 0.046 mol) into a flask, add 6 g of petroleum ether, cool to 0-5° C. with ice water, and add 6 g (0.05 mol) of thionyl chloride dropwise. After the dropwise addition, stir at 20-30°C for 3 hours to obtain a 2-butenoyl chloride reaction solution for use.
[0030] Put 6.2 grams (0.042mol) of N-ethyl-2-ethylaniline in another flask, add 6 grams of petroleum ether, then add 25g of 30% sodium hydroxide aqueous solution, keep at 10-20°C, and add dropwise under careful stirring The above 2-butenoyl chloride reaction solution was added dropwise within 40 minutes. Then react at 60-70°C until the reactant N-ethyl-2-methylaniline is basically completely reacted, about 8 hours.
[0031] According to the gas phase analysis, the product N-ethyl-N-(2'-ethylphenyl)-2-butenoyl content in the reactant was 95.8%, and the cis-trans ratio was 1.8:98.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com