A simple and efficient method for synthesizing 4-arylbutyric acid derivatives
A technology for aryl butyric acid and derivatives, which is applied in the field of simple and efficient synthesis of 4-aryl butyric acid derivatives, can solve the problems of too many additives, lengthy synthesis steps, harsh reaction conditions, etc., and solve the cumbersome steps of the synthesis process , high product yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Under air conditions, 0.2mmol N-(8-aminoquinoline) but-3-enamide, 0.4mmol phenyltrimethoxysilane, 0.02mmol palladium acetate, 0.4mmol copper fluoride, 2.0mmol acetic acid And 2.0mL tetrahydrofuran is added into the reaction vessel and mixed and stirred evenly; the reaction vessel is a pressure-resistant sealed tube containing a magnetic stirrer;
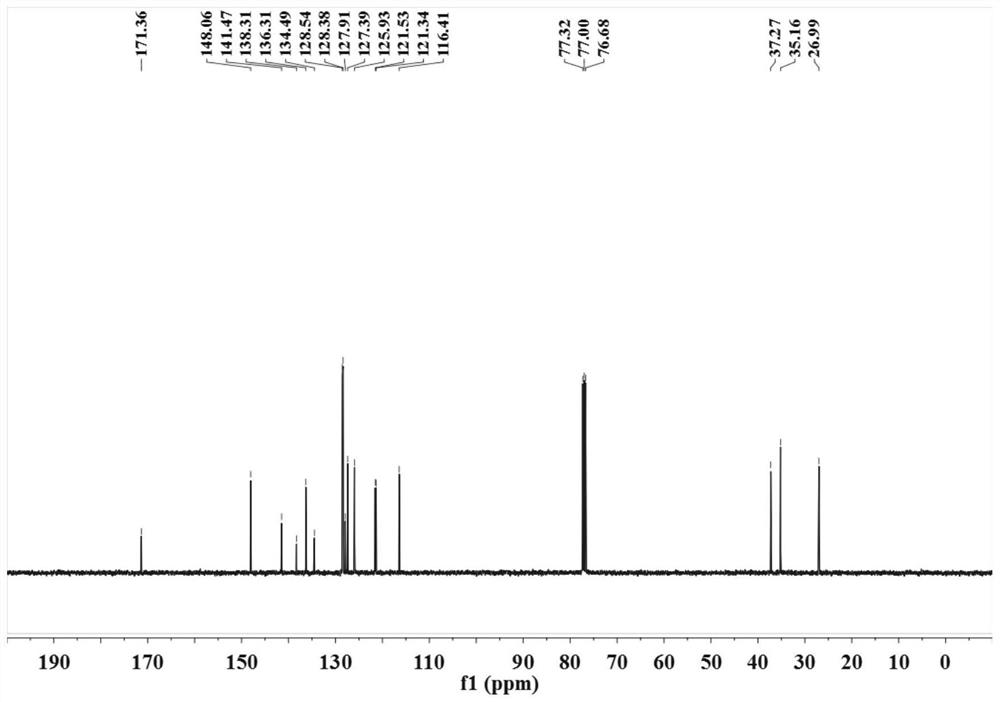
[0029] (2) After sealing the reaction vessel, put it into an oil bath at 100° C. to vigorously stir for 24 hours, TLC (developing agent is V 石油醚 :V 乙酸乙酯 =9:1) The detection substrate disappears and the reaction ends. The reaction solution was poured into saturated brine (15mL), extracted with dichloromethane (3×10mL), the combined organic phases were dried over anhydrous sodium sulfate, suction filtered, vacuum distillation, etc., and subjected to silica gel column chromatography (eluent for V 石油醚 :V 乙酸乙酯 =9:1) to obtain a colorless solid, the target product was 1 H NMR, 13 C NMR test proved that it was 4-arylbutanoi...
Embodiment 2
[0031] (1) Under air conditions, 0.2mmol N-(8-aminoquinoline) but-3-enamide, 0.4mmol 4-methylphenyltrimethoxysilane, 0.02mmol palladium acetate, 0.4mmol copper fluoride , 2.0mmol acetic acid and 2.0mL tetrahydrofuran were added to the reaction vessel and mixed and stirred evenly; the reaction vessel was a pressure-resistant sealed tube containing a magnetic stirrer;
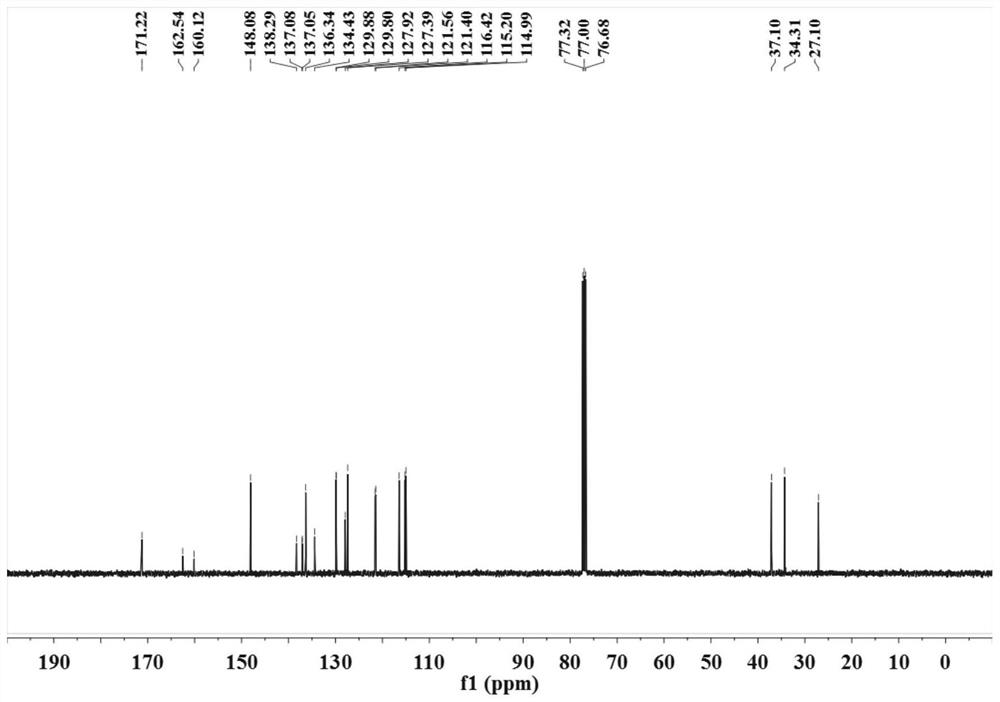
[0032] (2) After sealing the reaction vessel, put it into an oil bath at 100° C. to vigorously stir for 24 hours, TLC (developing agent is V 石油醚 :V 乙酸乙酯 =9:1) The detection substrate disappears and the reaction ends. The reaction solution was poured into saturated brine (15mL), extracted with dichloromethane (3×10mL), the combined organic phases were dried over anhydrous sodium sulfate, suction filtered, vacuum distillation, etc., and subjected to silica gel column chromatography (eluent for V 石油醚 :V 乙酸乙酯 =9:1) to obtain a colorless solid, the target compound was 1 H NMR, 13 C NMR test proved that it was 4-...
Embodiment 3
[0034] (1) Under air conditions, 0.2mmol N-(8-aminoquinoline) but-3-enamide, 0.4mmol 4-fluorophenyltrimethoxysilane, 0.02mmol palladium acetate, 0.4mmol copper fluoride, Add 2.0mmol acetic acid and 2.0mL tetrahydrofuran into the reaction vessel and mix well; the reaction vessel is a pressure-resistant sealed tube containing a magnetic stirrer;
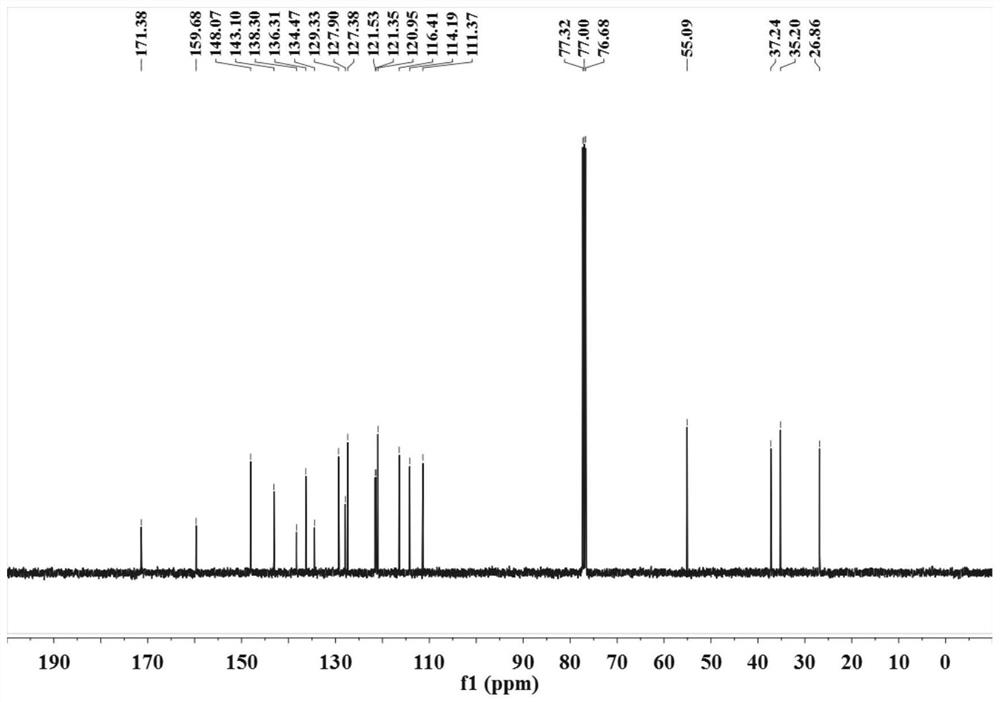
[0035] (2) After sealing the reaction vessel, put it into an oil bath at 100° C. to vigorously stir for 24 hours, TLC (developing agent is V 石油醚 :V 乙酸乙酯 =9:1) The detection substrate disappears and the reaction ends. The reaction solution was poured into saturated brine (15mL), extracted with dichloromethane (3×10mL), the combined organic phases were dried over anhydrous sodium sulfate, suction filtered, vacuum distillation, etc., and subjected to silica gel column chromatography (eluent for V 石油醚 :V 乙酸乙酯 =9:1) to obtain a colorless solid, after 1 H NMR, 13 C NMR test proved that it was 4-arylbutanoic acid derivative 3c, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com