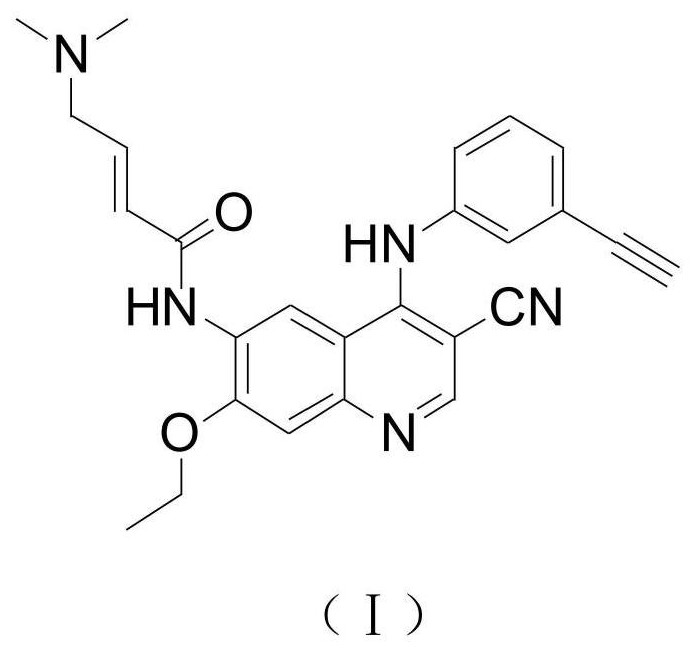
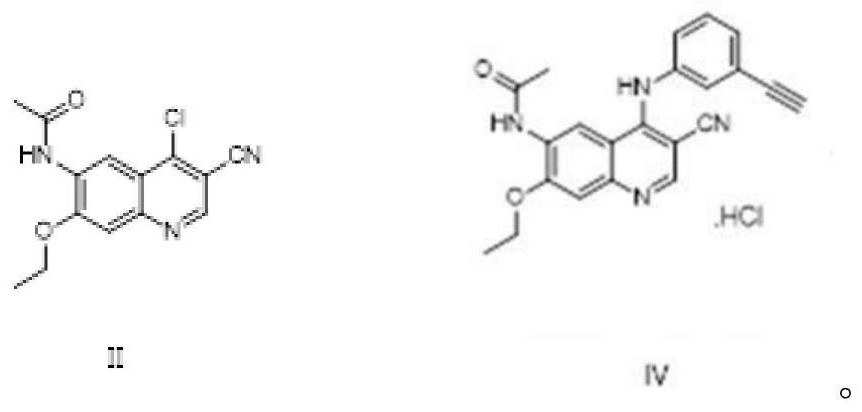
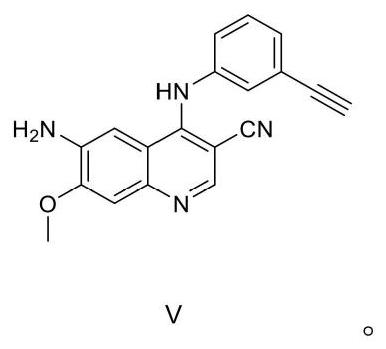
Preparation method of EGFR molecular targeting antitumor drug
A cyano and ethoxy technology, applied in antitumor drugs, drug combinations, organic chemistry, etc., can solve the problems of low conversion rate, difficult quality control, and high impurity content of compound IV
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0065] Embodiment 1-1: Preparation of Compound IV
[0066] In a 50L reactor, add 1.155kg N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl)acetamide, 18.48kg n-propanol, stir well; add 0.52kg m-aminophenylacetylene (the molar ratio of compound III to compound II is: 1.1:1); heat up to 90°C-95°C, react for 3 hours to reach the end, cool down to 0°C-5°C, centrifuge, wash the solid with n-propanol, dry to obtain the product 1.544kg, in the form of hydrochloride, the yield is 96%, and the HPLC test content is 99.58%.
Embodiment 1-2
[0067] Embodiment 1-2: the preparation of compound IV
[0068] In a 50L reactor, add 1.500kg N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl)acetamide, 18.00kg n-propanol, stir well; add 0.789kg m-aminophenylacetylene (the molar ratio of compound III to compound II is 1.3:1); heat up to 85°C to 90°C, react for 3 hours to reach the end, cool to 10°C to 15°C, centrifuge, wash the solid with n-propanol, and dry to obtain the product 2.015kg, the content is 99.46%, and the yield is 96%.
Embodiment 1-3
[0069] The preparation of embodiment 1-3 compound IV:
[0070] In a 50L reactor, add 1.00kg N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl)acetamide, 15.00kg n-propanol, stir well; add 0.325kg m-aminophenylacetylene (the molar ratio of compound III to compound II is: 0.8: 1); the temperature is raised to boiling reflux, and the reaction reaches the end point in 2.7 hours, the temperature is lowered to 0°C to 10°C, centrifuged, the solid is washed with n-propanol, and dried to obtain 1.336kg of product, The yield is 95%, and the content is 99.53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com