A kind of method of synthesizing chlorambucil
A technology for chlorambucil and n-butylphosphine tetrafluoroborate, which is applied in the field of synthesizing chlorambucil, can solve the problems of low product yield, complicated post-processing purification process, many synthesis steps and the like, and achieves the The reaction and post-treatment purification process is simple, the reaction region selectivity and yield are high, and the anti-tumor effect is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
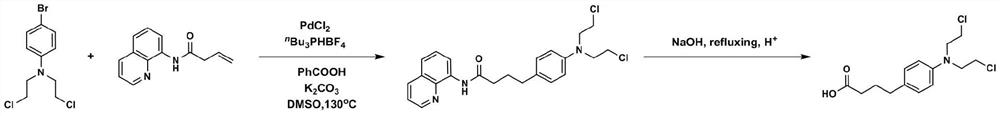
[0029] (1) Under an inert atmosphere, 4-bromo-N,N-bis(2-chloroethyl)aniline, N-(quinoline-8- Base) but-3-enamide, palladium chloride, tri-n-butylphosphine tetrafluoroborate, potassium carbonate, benzoic acid and dimethyl sulfoxide were added in a molar volume ratio of 0.1 mmol: 2 mL. Mix in the reaction vessel;
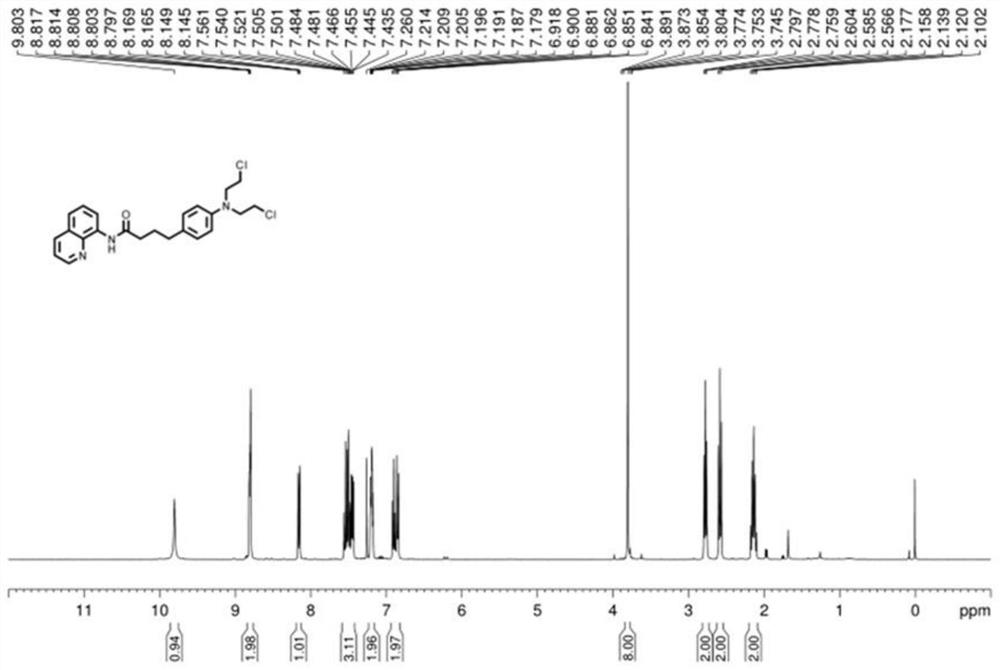
[0030] (2) The reaction vessel was placed in an oil bath at 135°C and vigorously stirred and reacted for 24 hours, and the reaction product was separated and purified by flushing a chromatographic silica gel column with petroleum ether in a ratio of 1:5 to ethyl acetate to obtain a compound with a leaving group. Detection confirmed that the compound was 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(quinolin-8-yl)butanamide;
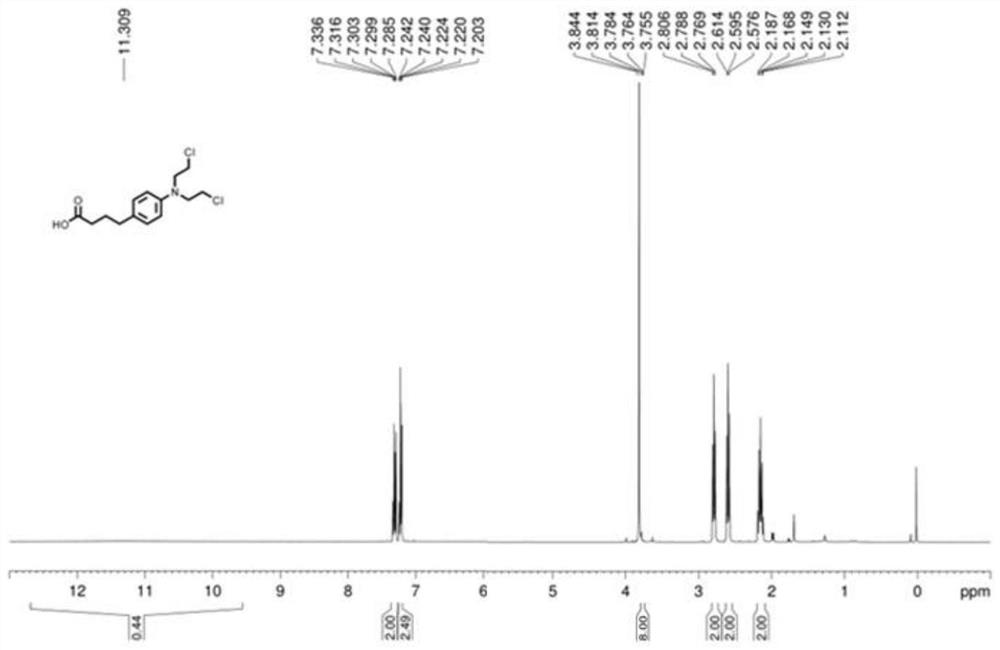
[0031] (3) After adding the compound with the leaving group to the aqueous solution of sodium hydroxide and heating under reflux for 12 hours, acidification was performed to obtain chlorambucil, and the yield of chlorambucil was 41%.
Embodiment 2
[0033] (1) Under an inert atmosphere, 4-bromo-N,N-bis(2-chloroethyl)aniline, N-(quinoline-8- Base) but-3-enamide, palladium chloride, tri-n-butylphosphine tetrafluoroborate, potassium carbonate, benzoic acid and dimethyl sulfoxide were added in a molar volume ratio of 0.1 mmol: 2 mL. Mix in the reaction vessel;
[0034] (2) The reaction vessel was placed in an oil bath at 135°C and vigorously stirred and reacted for 24 hours, and the reaction product was separated and purified by flushing a chromatographic silica gel column with petroleum ether in a ratio of 1:5 to ethyl acetate to obtain a compound with a leaving group. Detection confirmed that the compound was 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(quinolin-8-yl)butanamide.
[0035] (3) After adding the compound with the leaving group to the aqueous solution of sodium hydroxide and heating under reflux for 12 hours, acidification is performed to obtain chlorambucil, and the yield of chlorambucil is 6%.
Embodiment 3
[0037] (1) Under an inert atmosphere, 4-bromo-N,N-bis(2-chloroethyl)aniline, N-(quinoline-8- Base) but-3-enamide, palladium chloride, tri-n-butylphosphine tetrafluoroborate, potassium carbonate, benzoic acid and dimethyl sulfoxide were added in a molar volume ratio of 0.1 mmol: 2 mL. Mix in the reaction vessel;
[0038](2) The reaction vessel was placed in an oil bath at 135°C and vigorously stirred and reacted for 24 hours, and the reaction product was separated and purified by flushing a chromatographic silica gel column with petroleum ether in a ratio of 1:5 to ethyl acetate to obtain a compound with a leaving group. Detection confirmed that the compound was 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(quinolin-8-yl)butanamide.
[0039] (3) After adding the compound with the leaving group to the aqueous solution of sodium hydroxide and heating under reflux for 12 hours, acidification is performed to obtain chlorambucil, and the yield of chlorambucil is 9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com