A kind of preparation method of sodium phenylbutyrate
A sodium phenylbutyrate preparation technology, which is applied in the field of preparation of sodium phenylbutyrate, can solve the problems of low product yield, many synthesis steps, complicated post-treatment purification process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
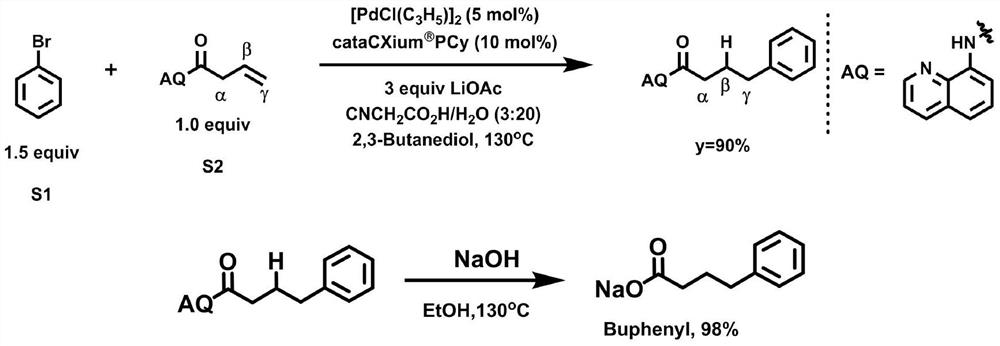
[0018] (1) Under an argon atmosphere, add the material and anhydrous 2,3-butanediol in a molar volume ratio of 0.1mmol:0.1mL to the reaction vessel and mix well; wherein, the related materials include a molar ratio of 1: 3:0.01:0.2:5:3:100 of N-(octaaminoquinoline)but-3-enamide, bromobenzene, allylpalladium(II) chloride dimer, 2-(dicyclohexylphosphine Acyl)-1-phenyl-1H-pyrrole, lithium acetate, cyanoacetic acid and water;
[0019] (2) Place the reaction vessel in an oil bath at 125°C and vigorously stir for 12 hours, and purify the reaction product through a silica gel column (flush the chromatography silica gel column with petroleum ether at a ratio of 1:20 to ethyl acetate), to obtain The compound is determined to be 4-phenyl-N-(quinolin-8-yl)butanamide through detection.
Embodiment 2
[0021] This embodiment is basically the same as Embodiment 1, the difference is:
[0022] In step (1), N-(octaaminoquinoline)but-3-enamide, bromobenzene, allylpalladium(II) chloride dimer, 2-(dicyclohexylphosphono)-1- The molar ratio of phenyl-1H-pyrrole, lithium acetate, cyanoacetic acid and water is 1:1:0.1:0.02:1:1:1;
[0023] In step (2), the temperature of the oil bath is 130°C.
Embodiment 3
[0025] This embodiment is basically the same as Embodiment 1, the difference is:
[0026] In step (1), N-(octaaminoquinoline)but-3-enamide, bromobenzene, allylpalladium(II) chloride dimer, 2-(dicyclohexylphosphono)-1- The molar ratio of phenyl-1H-pyrrole, lithium acetate, cyanoacetic acid and water is 1:2:0.05:0.1:3:2:50;
[0027] In step (2), the temperature of the oil bath is 135°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com